Three minutes to showtime, Gahl lumbers into the room and toward the podium. He’s spent the morning mapping out an ADA-compliant route for his patient, and he is sweating. “Does this work?” he asks into a dead microphone. “Of course not. This is the United States government. Nothing works.” (A government shutdown, ultimately averted, is believed to be imminent.) While he tries to diagnose the A/V failure, his teenage patient pilots a motorized wheelchair toward the front of the room, where his parents are fidgeting in their seats.
When the clock strikes nine, Gahl gives up on the microphone and decides to bellow instead: “Graham, is it okay if I ask you a few questions?”

The young man in the wheelchair nods. He’s wearing a Toronto Maple Leafs T-shirt, and his hair has been buzzed for an electroencephalogram.
“How old are you?”
There’s a ten-second pause that feels much longer. “Eighteen,” whispers Graham Tucker, eliciting audible sighs of relief from the audience. After some gentle coaxing from his mother, Tucker reveals that he’s come from Woodstock, the dairy capital of Canada. For a decade, no doctor could identify the disease ravaging his central nervous system. But that’s no longer the case.
Gahl heads the Undiagnosed Diseases Program, a clinic-of-last-resort established under the auspices of the National Human Genome Research Institute. His team specializes in genetic conditions affecting between one and 50 people worldwide. Using state-of-the-art genomic techniques, they routinely take on cases that have baffled top specialists: hair follicles that produce crystalline spikes, arteries that calcify into bone, neurons that begin failing at birth. Since 2008, they’ve managed to diagnose approximately 400 patients, publish roughly 200 articles in leading medical journals, and discover 30 new diseases, all on a shoestring budget. Though these diseases are rarely treatable, “a diagnosis is still everything,” says Ellen Macnamara, a genetic counselor with the UDP. A diagnosis means insurers are more likely to cover physical therapy and home care. Families can make informed decisions about the future, including whether to bring more children into the world. And after years of wandering alone, patients eventually find others like them, says Macnamara: “A diagnosis offers a home.”
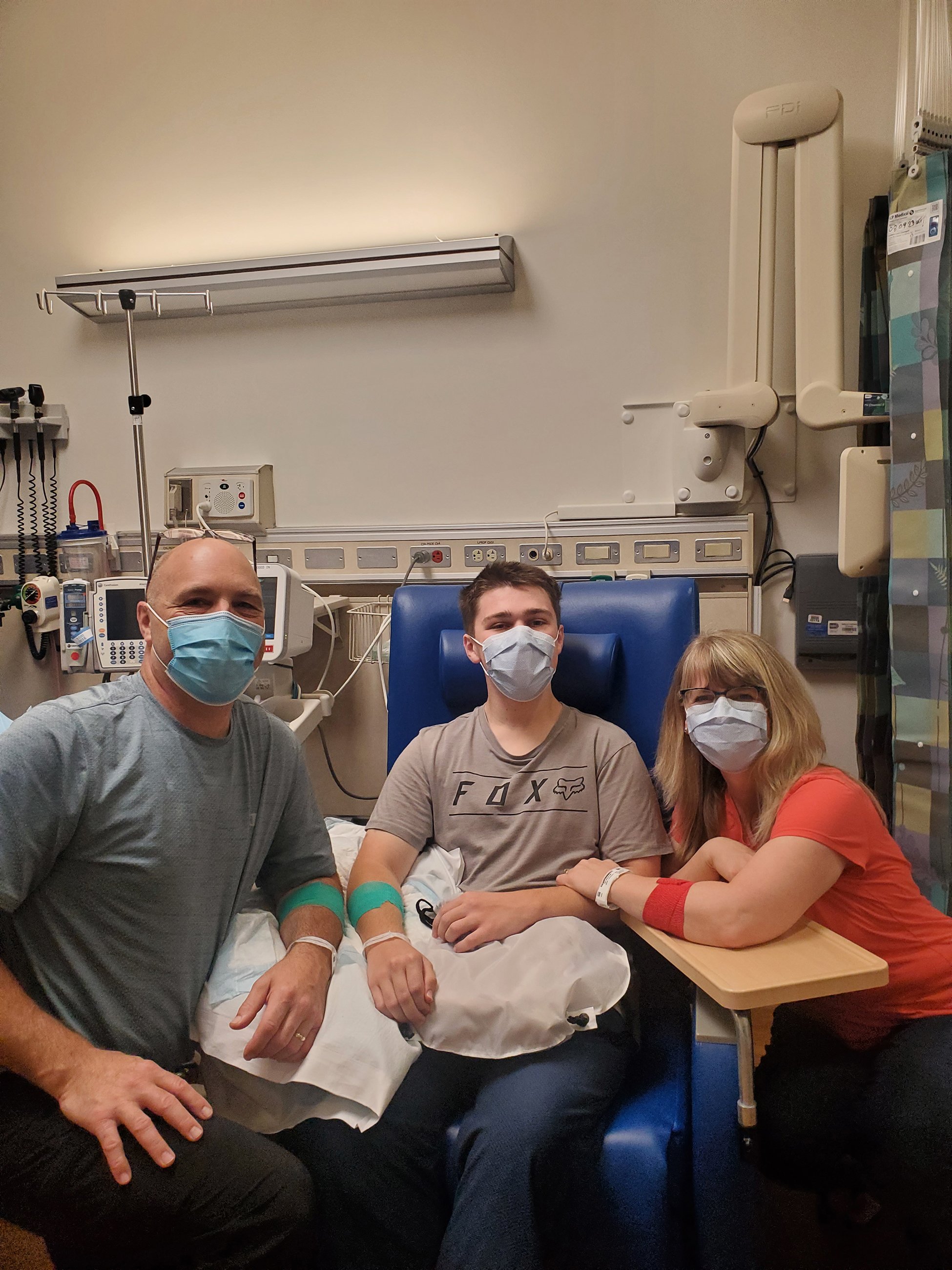
The trouble began at six months old. When Graham Tucker pushed Cheerios across his high-chair tray, his hands trembled. His parents, Susan and Jeff, figured he had a benign tremor like his grandfather. But something else was wrong: Graham didn’t walk or talk until he was 17 months old, and he was still using one-word phrases well into his second year. He was diagnosed with a common developmental delay in kindergarten, but his academic progress soon plateaued and his behavior grew erratic.
“Every step in the day was a battle,” Susan says. Graham refused all food that wasn’t crunchy. He couldn’t bear the feeling of compression, so his wardrobe consisted of a pair of Crocs and a loose mesh singlet hand-sewn by his mother. Car rides were particularly difficult, with short drives often spiraling into 90 minutes of screaming and biting.
In July 2011, three months before his seventh birthday, Graham went to his grandmother’s house for a family gathering. His older cousins took turns flipping and cannonballing off a diving board. As Graham watched from afar, his body went rigid and he fell straight back, “like a tree in the forest.” He lay on the ground for five seconds, frozen but awake, then stood up as if nothing had happened. By summer’s end, he was collapsing constantly—a short walk into a restaurant could trigger three or four episodes. After two hard hits to the head that Susan suspects left Graham with undiagnosed concussions, he began using a walker and then a wheelchair.
As the Tuckers navigated Canada’s health system, trying to figure out what was causing their son’s symptoms, they faced extraordinary wait times—18 months to see a developmental pediatrician, another 12 months for a pediatric neurologist. Ruling out obvious maladies, such as epilepsy or brain cancer, took years. Eventually, Susan and Jeff noticed a pattern in Graham’s episodes: They seemed to be triggered by any form of anxiety or alarm. If a rubber ball bounced his way, he’d collapse. If he walked into a busy place, he’d collapse. If someone said, “Watch out,” he’d collapse.
They consulted a child psychiatrist, who recommended blunting the panic with pharmaceuticals. For several years, they rode a never-ending carousel of antipsychotics, antidepressants, and benzodiazepines. None helped. In the run-up to a transatlantic flight, the psychiatrist recommended that Graham take three Valium tablets. This should be enough diazepam to tranquilize a professional wrestler—but Graham remained wide awake and enraged the entire trip from Scotland to Toronto. That was the last time he traveled by plane, and it was the last time his parents tried to medicate his mood. “We were sick and tired of him being a pin cushion,” Susan says.
The Tuckers continued turning over every stone, no matter how remote the chance of finding an answer. They contacted specialists in three countries about a list of increasingly esoteric conditions: rare epilepsies and dyskinesias; “fainting goat” and “stiff person” syndromes; hyperekplexia, a disease that causes people to freeze when startled. None fit the bill.
“When you’re managing negative behavior, people judge your parenting skills. That’s hard,” Susan says. “There were so many occasions where I wished we could say our son has an extremely rare genetic disorder and he struggles every day to cope with just about everything.”
In 2016, Canadian health authorities agreed to foot the bill for a genetic test known as an exome sequence. Though the exome is just 2 percent of all the genes in our DNA, it contains instructions for producing the proteins that keep us alive—85 percent of disease-causing mutations are found there. But that test, like all the others, came back negative. Eventually, the family’s longtime neurologist called the Tuckers in for a meeting. It was to admit defeat. He wouldn’t drop Graham as a patient—they could still come back for a check-in every six months. But they’d have to find their miracle elsewhere.
William Gahl works from a corner office, but only in the most literal sense. The lighting is dim. The draft is supernatural. The door requires routine oiling. The room is mostly desk, and the desk is mostly medical records, plus a 12-pack of Diet Mountain Dew, a Far Side calendar that hasn’t been updated in five days, and a computer monitor buttressed by two volumes of The Metabolic and Molecular Bases of Inherited Disease. His award-covered wall tells the story of an illustrious career—not in medicine but in over-70 softball. “I can’t hit for power anymore,” Gahl laments, feet kicked up on the desk. He can, however, provide medical attention when a fellow old-timer invariably drops in the summer heat.
Gahl began his four-decade career at NIH in 1981. After racing through his MD, PhD, and pediatric residency at the University of Wisconsin, he moved to Bethesda to train in the nascent science of clinical genetics. By the end of the decade, Gahl was running that fellowship program, as well as the human-genetics division of the National Institute of Child Health and Human Development. His rise was propelled by his work with cystinosis, a genetic disorder that causes an amino acid to build up inside the cells, eventually leading to kidney failure and death. When Gahl was just 31, he discovered the underlying enzyme deficiency, and later earned FDA approval for a life-extending drug that flushes out the excess cystine.
While Gahl climbed the ladder at NIH, an international consortium of scientists set out to catalog the 3 billion nucleotide base pairs that make up our DNA. The endeavor, dubbed the Human Genome Project, was preposterous. It would require the kind of international collaboration typically reserved for enormous particle accelerators and space stations—even a world-class lab would struggle to sequence more than a thousand base pairs a week. Some observers predicted it would be a historic boondoggle. Others believed it could “grant insight into human biology previously held only by God.”
It took $2.7 billion and 13 years of mind-numbing drudge work, but in 2003 the Human Genome Project re-leased a more-or-less-complete sequence of the 63,000 genes spread across our 23 chromosome pairs. For nearly a century, biologists had been exploring DNA with the equivalent of sextants and sunstones—it could take decades of trial and error to locate the gene they’d set out to find. Now, with a genetic map, they could navigate to a specific gene of interest, document its physiological function, then study how mutations affect that function.
Meanwhile, Gahl had been appoint-ed clinical director of the National Human Genome Research Institute, the NIH division tasked with integrating arcane genomic research into everyday medical care. Early on, he was approached by Stephen Groft, longtime head of the Office of Rare Disease Research. Groft had a problem: His budget was earmarked for patients with known diseases, but his voicemail was full of messages from people with undiagnosed or misdiagnosed conditions. In theory, genomics could reduce the guesswork involved in diagnosis, rapidly winnowing the possible causes of chronic illness. In practice, that dream would be hard to realize. Even basic sequencing was prohibitively expensive, and few physicians were trained to analyze genetic data. But if Gahl was interested in setting up a pilot program inside NIH, Groft could front $280,000 to get it up and running.
In 2008, Gahl hired two nurse practitioners, began calling in favors from colleagues who had a spare hour, and launched the first iteration of the Undiagnosed Diseases Program. The goal: to use the world-class sequencing technology at NIH to crack those difficult cases, find new diseases to study, and create a living blueprint for genomic medicine. His team has since grown to 40. They’ve taken on 1,600 cases from 20 countries and solved a quarter of them. Their typical patient has spent eight years searching for answers and has a medical chart that runs hundreds—sometimes thousands—of pages. Each week, one or two new patients travel to Bethesda to undergo a five-day diagnostic marathon offered at no cost. Though each visit is custom-tailored, it could plausibly include visits with a dozen specialists, plus 50-odd tests and examinations.
“The good news is that you’re going to get a year’s worth of evaluation in one week,” says Dr. Cynthia Tifft, who leads the UDP’s pediatric branch. “The bad news is that you’re going to get a year’s worth of evaluation in a week.”
Sometimes, diagnoses do not require any genetic testing. The patient could have a hard-to-detect cancer or unusual presentation of a not-so-rare disease. Maybe they’ve been exposed to a toxin. Years ago, a man from Texas was referred to the UDP with off-the-charts potassium levels, putting him at risk of cardiac arrest. After Gahl ordered a urine test, it was clear the man was consuming enormous quantities of potassium every day, far more than anyone could get from bananas alone. “The sheriff in his county called me up and asked why he was in Maryland,” Gahl says. “It turns out he embezzled $400,000.” The patient’s mother was deliberately poisoning him, hoping he could evade arrest if he was hospitalized.
When doctors suspect a disease has been caused by a mutation, they have access to an ever-growing arsenal of research tools. They can rapidly sequence a patient’s entire genome. They can do the same for microbes living inside their body. They can analyze RNA, the immediate product of DNA, to reveal whether any important genes have been turned off. They can scan for unusual patterns of inheritance, the sort that mostly exist as hypotheticals in textbooks. Best case, they’ll find a known genetic defect. More commonly, they’ll find dozens of what they call “variants of unknown significance,” which may or may not be damaging. Algorithms can cull the list of genes, and the most promising candidates can then be evaluated in various species of critters.

In an underground facility beneath the NIH campus are 9,000 aquarium tanks filled with half a million zebrafish. The striped swimmers are no larger than a paper clip, yet the genetic overlap between them and humans is about 70 percent. A female zebrafish can release hundreds of eggs into the water every week, and fertilized embryos begin to form visible organs within 24 hours. All of these characteristics make them ideal models for studying mutations. If Gahl suspects, for example, that a variant is causing brain damage, he can ask the lab to replicate that mutation inside an analogous zebrafish gene. If similar symptoms emerge, they’ve probably identified the culprit.
The program’s greatest asset, however, is time. A UDP doctor devotes an entire week to one patient. That leaves plenty of time to pore over Dostoevskian medical records, thumb through obscure scientific journals, and tap into the wealth of hyper-specific expertise available at NIH. “It’s not like we’re smarter than everyone else. If you think about a [primary-care physician] seeing 20 or 40 people a day, they don’t have a lot of time to navel-gaze about each individual person they see,” says Dr. David Adams, a clinical geneticist and codirector of the UDP. “Not to get too sappy, but this is made possible by the American public funding this institution. It couldn’t happen if we were relying on insurance reimbursement.”
The Tuckers were accepted into the UDP in late 2019. But one thing after another delayed their visit to Bethesda. Pandemic-era border restrictions prevented them from entering the United States. Jeff and Susan lost their jobs. Jeff’s mother was killed by a runaway rice truck in Kenya. Gahl ordered a remote blood draw for the family. This was far from ideal, but it gave his team some genetic data to digest while things settled down.
In July 2022, Susan received an email from Don Hadley, a genetic counselor at NIH. He had news. The bioinformatics team had found two distinct mutations—one passed from each parent—in WARS2, a gene essential to mitochondria, the power plants found in every human cell. When mitochondria go haywire, energy-hungry nerve cells can starve to death, causing severe movement disorders.
“I cried—I’m going to cry right now,” Susan tells me. For years, she had been haunted by the memory of dropping her sunscreen-covered baby on a pool deck. “I’ve lived with that guilt his entire life. It meant so much to hear somebody say, ‘There’s nothing you did, no medication you gave him, no vaccine that could have caused this. It’s just his genes.’ ”

The lead physician on Graham’s case is Camilo Toro, a jovial neurologist with salt-and-pepper hair and a knack for explaining things at a fifth-grade level. “I come in on the first day of the week and say hello to the patients. They’re incredibly happy to see me,” Gahl says. “Then Camilo takes care of them. By the end of the week, I’m dog shit. It’s only Camilo.”
Before joining the UDP in 2009, Toro specialized in Parkinson’s and other neurodegenerative diseases. He’s well acquainted with Graham’s condition, known as childhood-onset parkinsonism dystonia 3, because he’s seen it twice before at NIH. He was among the authors who first described the disease in a medical journal. Since then, 17 additional cases have been documented around the world. Though symptoms vary slightly, the underlying problem is the same.
Inside every human cell are millions of factories, called ribosomes, where amino acids are assembled into complex proteins. Those proteins are shipped throughout the cell, where they’re used to reinforce structures, transport other molecules, and sustain the chemical reactions that keep us alive. All production is governed by the DNA, which functions as a corporate handbook: It contains blueprints for thousands of different proteins, plus strict guidelines for factory operations.
The WARS2 gene contains instructions for staffing the teams that produce proteins destined for the mitochondria. Graham’s mutations have left typos throughout that page of the handbook. Not enough workers have been hired, the assembly lines have fallen into disarray, and proteins are leaving the factory missing key components. Without functional proteins, the mitochondria struggle to produce sufficient power, causing rolling blackouts in areas of Graham’s brain.
“There’s still a lot to understand about the biology of it,” Toro says. Though Graham was diagnosed from afar, Toro has brought him to Bethesda for further evaluation and to collect cells to study in the lab. Ideally, Toro would extract tissue from Graham’s brain, then scour those specialized cells for chemical markers of mitochondrial dysfunction. But poking around inside the skull is high-risk. Instead, Toro plans to use a curious form of alchemy.
Embryonic stem cells are highly coveted by biomedical scientists. The reason is twofold. They can be transformed into any one of the 220 cell types found in the human body. They can also self-replicate in perpetuity, offering a near-infinite supply of research material. But they’re also difficult to source and highly controversial among pro-life politicians and activists. In 2007, however, researchers from Kyoto University discovered a method for reverting human fibroblasts, common cells found throughout the skin, back to an embryonic state. For most labs, these are as good as the real thing.
In practice, this means Toro can take a simple skin biopsy from Graham, then use it to create a self-sustaining supply of neurons, all preloaded with the WARS2 mutation. In the coming months and years, those cells will be used to study the defect, as well as potential therapies—not only for Graham but potentially for millions of people with Parkinson’s. “If we unravel the mystery, it will tell us a lot about the role of tRNA synthetases and mitochondrial dysfunction in Parkinson’s disease. That’s a really, really hot topic,” Toro says. “[Graham] is a window into why those neurons are dying and what we can do to help.”
The only thing harder than diagnosing rare diseases is treating them. Fewer than 10 percent have an approved therapy, and the figure is even lower for the ultra-rare conditions the UDP sees. Even if you understand the biology of a disease, bringing a new drug to market can take decades of toil and many millions of dollars. Federal tax incentives exist for pharmaceutical companies to take a chance on “orphan” drugs, but even so, there’s little interest in developing a product intended for 60 people. The onus tends to fall on physician-scientists and advocacy groups.
“Money talks in this environment, unfortunately,” Tifft says. She specializes in GM-1 and GM-2 gangliosidosis, fatal neurodegenerative diseases that usually strike in early childhood. Her hair is violet, the signature color of the National Tay-Sachs and Allied Diseases Association. “A few years ago, my research nurse talked me into dyeing my hair for one of their annual family meetings. I walked in, and the kids thought it was hysterical. Now it’s purple until we find a cure,” she tells me.
It might not be purple much longer. Tifft is leading a gene-therapy trial for GM-1. Her drug is a harmless virus, engineered to deliver a functional copy of the GLB1 gene throughout the central nervous system. When tested in cats, it boosted their lifespan from ten months to five years. Since then, Tifft has treated 12 children. Almost all show no sign of disease progression. Though the early results are encouraging, it has taken more than a decade to get that far. There’s no “plug and play” gene-therapy system approved by the FDA: Even when a viral vector has been well studied and well tolerated in previous trials, regulators require a full portfolio of costly safety studies before it can be tested in a new disease. For independent researchers, those hurdles are often insurmountable.
The relationship between rare-disease specialists and the FDA has always been fraught. The agency is inflexible by design, and its leaders are loath to make exceptions. That tends to frustrate doctors who work with exceptional cases. In 2007, Gahl diagnosed a baby girl with Canavan disease, a fatal white-matter disorder that progresses rapidly from birth. In his estimation, they had 12 weeks to treat her. There was already a chemical, deemed safe by the FDA for other uses, that would probably correct her myelin deficiency. When he submitted an emergency-use request, he received a four-page form letter requesting a series of studies that would take at least a year to complete. They had no choice but to watch the child die. “This job isn’t for everybody,” he tells me.
It isn’t easy waging war against mutations, an inherent part of evolution. The casualties are senseless, largely inflicted by chance. Stewing on failure can be a recipe for burnout. But the job also has a way of demanding obsession, because many cases remain open for years.
Francis Rossignol, the youngest physician on the UDP staff, is still fixated on a case from three years ago, one of his first. “Her clinical presentation is the most generic possible: fatigue and vomiting. She fits a diagnosis, but we cannot find any variants in the genes known to be associated with it,” he says. “Those nag at you. You’re close to something but not there yet, and you’re not able to figure out what next step will bring you there.”
Occasionally, a new lead will emerge from revisiting an old chart every few months or diagramming the same biochemical pathway over and over. But more often, they must wait—for gene-function studies to pan out, for similar cases to emerge in the database, for sequencing technology to improve. Just recently, a patient was diagnosed a full decade after intake. “One of our attending physicians used to say it’s like searching a parking lot around a light post, but that’s not where you dropped your keys,” says Ellen Macnamara. “You just keep looking. The answer is out there.”
Graham’s disease can’t be cured yet. But it can be managed for a time. A few months ago, Toro showed me a video on his phone: A young man with the same WARS2 mutation is doubled over in bed, frozen by intense dystonic spasms. His golden retriever is inconsolable, jumping around at his feet and barking for help. In another clip, the same man is walking upright and shooting free-throws with textbook form. The difference is dopamine, supplemented through a drug called levodopa. It doesn’t fix the underlying deficiency, but for many people with parkinsonian disorders, it can help alleviate tremors, stiffness, and muscle spasms.
Shortly after he was diagnosed, Graham began his penultimate year of public school. He also began taking daily levodopa. By the time he returned from winter break, he was walking around the house. “We hadn’t seen him walk on his own in ten years,” Susan tells me in an inpatient suite at the Clinical Center. Graham can’t cover long distances—his muscles are too rigid, tendons too short, arches too high—so he still uses his wheelchair or walker in public. Even so, he’s “racing” on the walker, putting in five laps around his school’s campus every day.
With his newfound confidence, Graham is exploring interests outside the house. He volunteers with a youth group. He recently joined a basketball team through the Special Olympics. A game against the Woodstock Fire Department is fast approaching, and Graham has been working on his fundamentals. “I jumped out of the chair and grabbed the ball the other day,” he tells anyone willing to listen.
“I just want him to be happy,” Susan says. “I want him to have things in his life that give him some fulfillment, for him to feel like he has a purpose and isn’t just putting in time.”
No doctor can say exactly how Graham’s disease will progress. As he ages, his motor function could further deteriorate. And the effectiveness of the levodopa will almost certainly wane within a few years. Eventually, they’ll need to consider deep brain stimulation, a permanent electrical implant in the basal ganglia that helps regulate movement. But for now, they’re reveling in the miracle of a slightly more ordinary life. Graham is eating meatballs. He’s getting dressed in five minutes instead of an hour. And he’s finally made peace with his oldest enemy: shoes.
Toward the end of our chat, he points to his feet and grins. “Look,” he says. His rubber clogs are gone. In their place are black high-top sneakers, the kind every high-school boy has coveted at some point. “They have laces.”
It’s 8:53 AM, and Dr. William Gahl is dangerously close to pissing off the fire marshal again.
A legion of young people has come to hear the 73-year-old geneticist present his team’s latest breakthrough. Medical residents are crowding the exits. Research interns with tiny tattoos and giant water bottles are getting bounced at the door. Gahl usually lectures in a 260-seat auditorium in the NIH Clinical Center. This week, however, a conference has exiled him to a windowless classroom in the basement. Everybody is trying to make it work.
Three minutes to showtime, Gahl lumbers into the room and toward the podium. He’s spent the morning mapping out an ADA-compliant route for his patient, and he is sweating. “Does this work?” he asks into a dead microphone. “Of course not. This is the United States government. Nothing works.” (A government shutdown, ultimately averted, is believed to be imminent.) While he tries to diagnose the A/V failure, his teenage patient pilots a motorized wheelchair toward the front of the room, where his parents are fidgeting in their seats.
When the clock strikes nine, Gahl gives up on the microphone and decides to bellow instead: “Graham, is it okay if I ask you a few questions?”

The young man in the wheelchair nods. He’s wearing a Toronto Maple Leafs T-shirt, and his hair has been buzzed for an electroencephalogram.
“How old are you?”
There’s a ten-second pause that feels much longer. “Eighteen,” whispers Graham Tucker, eliciting audible sighs of relief from the audience. After some gentle coaxing from his mother, Tucker reveals that he’s come from Woodstock, the dairy capital of Canada. For a decade, no doctor could identify the disease ravaging his central nervous system. But that’s no longer the case.
Gahl heads the Undiagnosed Diseases Program, a clinic-of-last-resort established under the auspices of the National Human Genome Research Institute. His team specializes in genetic conditions affecting between one and 50 people worldwide. Using state-of-the-art genomic techniques, they routinely take on cases that have baffled top specialists: hair follicles that produce crystalline spikes, arteries that calcify into bone, neurons that begin failing at birth. Since 2008, they’ve managed to diagnose approximately 400 patients, publish roughly 200 articles in leading medical journals, and discover 30 new diseases, all on a shoestring budget. Though these diseases are rarely treatable, “a diagnosis is still everything,” says Ellen Macnamara, a genetic counselor with the UDP. A diagnosis means insurers are more likely to cover physical therapy and home care. Families can make informed decisions about the future, including whether to bring more children into the world. And after years of wandering alone, patients eventually find others like them, says Macnamara: “A diagnosis offers a home.”
The trouble began at six months old. When Graham Tucker pushed Cheerios across his high-chair tray, his hands trembled. His parents, Susan and Jeff, figured he had a benign tremor like his grandfather. But something else was wrong: Graham didn’t walk or talk until he was 17 months old, and he was still using one-word phrases well into his second year. He was diagnosed with a common developmental delay in kindergarten, but his academic progress soon plateaued and his behavior grew erratic.
“Every step in the day was a battle,” Susan says. Graham refused all food that wasn’t crunchy. He couldn’t bear the feeling of compression, so his wardrobe consisted of a pair of Crocs and a loose mesh singlet hand-sewn by his mother. Car rides were particularly difficult, with short drives often spiraling into 90 minutes of screaming and biting.
In July 2011, three months before his seventh birthday, Graham went to his grandmother’s house for a family gathering. His older cousins took turns flipping and cannonballing off a diving board. As Graham watched from afar, his body went rigid and he fell straight back, “like a tree in the forest.” He lay on the ground for five seconds, frozen but awake, then stood up as if nothing had happened. By summer’s end, he was collapsing constantly—a short walk into a restaurant could trigger three or four episodes. After two hard hits to the head that Susan suspects left Graham with undiagnosed concussions, he began using a walker and then a wheelchair.
As the Tuckers navigated Canada’s health system, trying to figure out what was causing their son’s symptoms, they faced extraordinary wait times—18 months to see a developmental pediatrician, another 12 months for a pediatric neurologist. Ruling out obvious maladies, such as epilepsy or brain cancer, took years. Eventually, Susan and Jeff noticed a pattern in Graham’s episodes: They seemed to be triggered by any form of anxiety or alarm. If a rubber ball bounced his way, he’d collapse. If he walked into a busy place, he’d collapse. If someone said, “Watch out,” he’d collapse.
They consulted a child psychiatrist, who recommended blunting the panic with pharmaceuticals. For several years, they rode a never-ending carousel of antipsychotics, antidepressants, and benzodiazepines. None helped. In the run-up to a transatlantic flight, the psychiatrist recommended that Graham take three Valium tablets. This should be enough diazepam to tranquilize a professional wrestler—but Graham remained wide awake and enraged the entire trip from Scotland to Toronto. That was the last time he traveled by plane, and it was the last time his parents tried to medicate his mood. “We were sick and tired of him being a pin cushion,” Susan says.
The Tuckers continued turning over every stone, no matter how remote the chance of finding an answer. They contacted specialists in three countries about a list of increasingly esoteric conditions: rare epilepsies and dyskinesias; “fainting goat” and “stiff person” syndromes; hyperekplexia, a disease that causes people to freeze when startled. None fit the bill.
“When you’re managing negative behavior, people judge your parenting skills. That’s hard,” Susan says. “There were so many occasions where I wished we could say our son has an extremely rare genetic disorder and he struggles every day to cope with just about everything.”
In 2016, Canadian health authorities agreed to foot the bill for a genetic test known as an exome sequence. Though the exome is just 2 percent of all the genes in our DNA, it contains instructions for producing the proteins that keep us alive—85 percent of disease-causing mutations are found there. But that test, like all the others, came back negative. Eventually, the family’s longtime neurologist called the Tuckers in for a meeting. It was to admit defeat. He wouldn’t drop Graham as a patient—they could still come back for a check-in every six months. But they’d have to find their miracle elsewhere.
William Gahl works from a corner office, but only in the most literal sense. The lighting is dim. The draft is supernatural. The door requires routine oiling. The room is mostly desk, and the desk is mostly medical records, plus a 12-pack of Diet Mountain Dew, a Far Side calendar that hasn’t been updated in five days, and a computer monitor buttressed by two volumes of The Metabolic and Molecular Bases of Inherited Disease. His award-covered wall tells the story of an illustrious career—not in medicine but in over-70 softball. “I can’t hit for power anymore,” Gahl laments, feet kicked up on the desk. He can, however, provide medical attention when a fellow old-timer invariably drops in the summer heat.
Gahl began his four-decade career at NIH in 1981. After racing through his MD, PhD, and pediatric residency at the University of Wisconsin, he moved to Bethesda to train in the nascent science of clinical genetics. By the end of the decade, Gahl was running that fellowship program, as well as the human-genetics division of the National Institute of Child Health and Human Development. His rise was propelled by his work with cystinosis, a genetic disorder that causes an amino acid to build up inside the cells, eventually leading to kidney failure and death. When Gahl was just 31, he discovered the underlying enzyme deficiency, and later earned FDA approval for a life-extending drug that flushes out the excess cystine.
While Gahl climbed the ladder at NIH, an international consortium of scientists set out to catalog the 3 billion nucleotide base pairs that make up our DNA. The endeavor, dubbed the Human Genome Project, was preposterous. It would require the kind of international collaboration typically reserved for enormous particle accelerators and space stations—even a world-class lab would struggle to sequence more than a thousand base pairs a week. Some observers predicted it would be a historic boondoggle. Others believed it could “grant insight into human biology previously held only by God.”
It took $2.7 billion and 13 years of mind-numbing drudge work, but in 2003 the Human Genome Project re-leased a more-or-less-complete sequence of the 63,000 genes spread across our 23 chromosome pairs. For nearly a century, biologists had been exploring DNA with the equivalent of sextants and sunstones—it could take decades of trial and error to locate the gene they’d set out to find. Now, with a genetic map, they could navigate to a specific gene of interest, document its physiological function, then study how mutations affect that function.
Meanwhile, Gahl had been appoint-ed clinical director of the National Human Genome Research Institute, the NIH division tasked with integrating arcane genomic research into everyday medical care. Early on, he was approached by Stephen Groft, longtime head of the Office of Rare Disease Research. Groft had a problem: His budget was earmarked for patients with known diseases, but his voicemail was full of messages from people with undiagnosed or misdiagnosed conditions. In theory, genomics could reduce the guesswork involved in diagnosis, rapidly winnowing the possible causes of chronic illness. In practice, that dream would be hard to realize. Even basic sequencing was prohibitively expensive, and few physicians were trained to analyze genetic data. But if Gahl was interested in setting up a pilot program inside NIH, Groft could front $280,000 to get it up and running.
In 2008, Gahl hired two nurse practitioners, began calling in favors from colleagues who had a spare hour, and launched the first iteration of the Undiagnosed Diseases Program. The goal: to use the world-class sequencing technology at NIH to crack those difficult cases, find new diseases to study, and create a living blueprint for genomic medicine. His team has since grown to 40. They’ve taken on 1,600 cases from 20 countries and solved a quarter of them. Their typical patient has spent eight years searching for answers and has a medical chart that runs hundreds—sometimes thousands—of pages. Each week, one or two new patients travel to Bethesda to undergo a five-day diagnostic marathon offered at no cost. Though each visit is custom-tailored, it could plausibly include visits with a dozen specialists, plus 50-odd tests and examinations.
“The good news is that you’re going to get a year’s worth of evaluation in one week,” says Dr. Cynthia Tifft, who leads the UDP’s pediatric branch. “The bad news is that you’re going to get a year’s worth of evaluation in a week.”
Sometimes, diagnoses do not require any genetic testing. The patient could have a hard-to-detect cancer or unusual presentation of a not-so-rare disease. Maybe they’ve been exposed to a toxin. Years ago, a man from Texas was referred to the UDP with off-the-charts potassium levels, putting him at risk of cardiac arrest. After Gahl ordered a urine test, it was clear the man was consuming enormous quantities of potassium every day, far more than anyone could get from bananas alone. “The sheriff in his county called me up and asked why he was in Maryland,” Gahl says. “It turns out he embezzled $400,000.” The patient’s mother was deliberately poisoning him, hoping he could evade arrest if he was hospitalized.
When doctors suspect a disease has been caused by a mutation, they have access to an ever-growing arsenal of research tools. They can rapidly sequence a patient’s entire genome. They can do the same for microbes living inside their body. They can analyze RNA, the immediate product of DNA, to reveal whether any important genes have been turned off. They can scan for unusual patterns of inheritance, the sort that mostly exist as hypotheticals in textbooks. Best case, they’ll find a known genetic defect. More commonly, they’ll find dozens of what they call “variants of unknown significance,” which may or may not be damaging. Algorithms can cull the list of genes, and the most promising candidates can then be evaluated in various species of critters.

In an underground facility beneath the NIH campus are 9,000 aquarium tanks filled with half a million zebrafish. The striped swimmers are no larger than a paper clip, yet the genetic overlap between them and humans is about 70 percent. A female zebrafish can release hundreds of eggs into the water every week, and fertilized embryos begin to form visible organs within 24 hours. All of these characteristics make them ideal models for studying mutations. If Gahl suspects, for example, that a variant is causing brain damage, he can ask the lab to replicate that mutation inside an analogous zebrafish gene. If similar symptoms emerge, they’ve probably identified the culprit.
The program’s greatest asset, however, is time. A UDP doctor devotes an entire week to one patient. That leaves plenty of time to pore over Dostoevskian medical records, thumb through obscure scientific journals, and tap into the wealth of hyper-specific expertise available at NIH. “It’s not like we’re smarter than everyone else. If you think about a [primary-care physician] seeing 20 or 40 people a day, they don’t have a lot of time to navel-gaze about each individual person they see,” says Dr. David Adams, a clinical geneticist and codirector of the UDP. “Not to get too sappy, but this is made possible by the American public funding this institution. It couldn’t happen if we were relying on insurance reimbursement.”
The Tuckers were accepted into the UDP in late 2019. But one thing after another delayed their visit to Bethesda. Pandemic-era border restrictions prevented them from entering the United States. Jeff and Susan lost their jobs. Jeff’s mother was killed by a runaway rice truck in Kenya. Gahl ordered a remote blood draw for the family. This was far from ideal, but it gave his team some genetic data to digest while things settled down.
In July 2022, Susan received an email from Don Hadley, a genetic counselor at NIH. He had news. The bioinformatics team had found two distinct mutations—one passed from each parent—in WARS2, a gene essential to mitochondria, the power plants found in every human cell. When mitochondria go haywire, energy-hungry nerve cells can starve to death, causing severe movement disorders.
“I cried—I’m going to cry right now,” Susan tells me. For years, she had been haunted by the memory of dropping her sunscreen-covered baby on a pool deck. “I’ve lived with that guilt his entire life. It meant so much to hear somebody say, ‘There’s nothing you did, no medication you gave him, no vaccine that could have caused this. It’s just his genes.’ ”

The lead physician on Graham’s case is Camilo Toro, a jovial neurologist with salt-and-pepper hair and a knack for explaining things at a fifth-grade level. “I come in on the first day of the week and say hello to the patients. They’re incredibly happy to see me,” Gahl says. “Then Camilo takes care of them. By the end of the week, I’m dog shit. It’s only Camilo.”
Before joining the UDP in 2009, Toro specialized in Parkinson’s and other neurodegenerative diseases. He’s well acquainted with Graham’s condition, known as childhood-onset parkinsonism dystonia 3, because he’s seen it twice before at NIH. He was among the authors who first described the disease in a medical journal. Since then, 17 additional cases have been documented around the world. Though symptoms vary slightly, the underlying problem is the same.
Inside every human cell are millions of factories, called ribosomes, where amino acids are assembled into complex proteins. Those proteins are shipped throughout the cell, where they’re used to reinforce structures, transport other molecules, and sustain the chemical reactions that keep us alive. All production is governed by the DNA, which functions as a corporate handbook: It contains blueprints for thousands of different proteins, plus strict guidelines for factory operations.
The WARS2 gene contains instructions for staffing the teams that produce proteins destined for the mitochondria. Graham’s mutations have left typos throughout that page of the handbook. Not enough workers have been hired, the assembly lines have fallen into disarray, and proteins are leaving the factory missing key components. Without functional proteins, the mitochondria struggle to produce sufficient power, causing rolling blackouts in areas of Graham’s brain.
“There’s still a lot to understand about the biology of it,” Toro says. Though Graham was diagnosed from afar, Toro has brought him to Bethesda for further evaluation and to collect cells to study in the lab. Ideally, Toro would extract tissue from Graham’s brain, then scour those specialized cells for chemical markers of mitochondrial dysfunction. But poking around inside the skull is high-risk. Instead, Toro plans to use a curious form of alchemy.
Embryonic stem cells are highly coveted by biomedical scientists. The reason is twofold. They can be transformed into any one of the 220 cell types found in the human body. They can also self-replicate in perpetuity, offering a near-infinite supply of research material. But they’re also difficult to source and highly controversial among pro-life politicians and activists. In 2007, however, researchers from Kyoto University discovered a method for reverting human fibroblasts, common cells found throughout the skin, back to an embryonic state. For most labs, these are as good as the real thing.
In practice, this means Toro can take a simple skin biopsy from Graham, then use it to create a self-sustaining supply of neurons, all preloaded with the WARS2 mutation. In the coming months and years, those cells will be used to study the defect, as well as potential therapies—not only for Graham but potentially for millions of people with Parkinson’s. “If we unravel the mystery, it will tell us a lot about the role of tRNA synthetases and mitochondrial dysfunction in Parkinson’s disease. That’s a really, really hot topic,” Toro says. “[Graham] is a window into why those neurons are dying and what we can do to help.”
The only thing harder than diagnosing rare diseases is treating them. Fewer than 10 percent have an approved therapy, and the figure is even lower for the ultra-rare conditions the UDP sees. Even if you understand the biology of a disease, bringing a new drug to market can take decades of toil and many millions of dollars. Federal tax incentives exist for pharmaceutical companies to take a chance on “orphan” drugs, but even so, there’s little interest in developing a product intended for 60 people. The onus tends to fall on physician-scientists and advocacy groups.
“Money talks in this environment, unfortunately,” Tifft says. She specializes in GM-1 and GM-2 gangliosidosis, fatal neurodegenerative diseases that usually strike in early childhood. Her hair is violet, the signature color of the National Tay-Sachs and Allied Diseases Association. “A few years ago, my research nurse talked me into dyeing my hair for one of their annual family meetings. I walked in, and the kids thought it was hysterical. Now it’s purple until we find a cure,” she tells me.
It might not be purple much longer. Tifft is leading a gene-therapy trial for GM-1. Her drug is a harmless virus, engineered to deliver a functional copy of the GLB1 gene throughout the central nervous system. When tested in cats, it boosted their lifespan from ten months to five years. Since then, Tifft has treated 12 children. Almost all show no sign of disease progression. Though the early results are encouraging, it has taken more than a decade to get that far. There’s no “plug and play” gene-therapy system approved by the FDA: Even when a viral vector has been well studied and well tolerated in previous trials, regulators require a full portfolio of costly safety studies before it can be tested in a new disease. For independent researchers, those hurdles are often insurmountable.
The relationship between rare-disease specialists and the FDA has always been fraught. The agency is inflexible by design, and its leaders are loath to make exceptions. That tends to frustrate doctors who work with exceptional cases. In 2007, Gahl diagnosed a baby girl with Canavan disease, a fatal white-matter disorder that progresses rapidly from birth. In his estimation, they had 12 weeks to treat her. There was already a chemical, deemed safe by the FDA for other uses, that would probably correct her myelin deficiency. When he submitted an emergency-use request, he received a four-page form letter requesting a series of studies that would take at least a year to complete. They had no choice but to watch the child die. “This job isn’t for everybody,” he tells me.
It isn’t easy waging war against mutations, an inherent part of evolution. The casualties are senseless, largely inflicted by chance. Stewing on failure can be a recipe for burnout. But the job also has a way of demanding obsession, because many cases remain open for years.
Francis Rossignol, the youngest physician on the UDP staff, is still fixated on a case from three years ago, one of his first. “Her clinical presentation is the most generic possible: fatigue and vomiting. She fits a diagnosis, but we cannot find any variants in the genes known to be associated with it,” he says. “Those nag at you. You’re close to something but not there yet, and you’re not able to figure out what next step will bring you there.”
Occasionally, a new lead will emerge from revisiting an old chart every few months or diagramming the same biochemical pathway over and over. But more often, they must wait—for gene-function studies to pan out, for similar cases to emerge in the database, for sequencing technology to improve. Just recently, a patient was diagnosed a full decade after intake. “One of our attending physicians used to say it’s like searching a parking lot around a light post, but that’s not where you dropped your keys,” says Ellen Macnamara. “You just keep looking. The answer is out there.”
Graham’s disease can’t be cured yet. But it can be managed for a time. A few months ago, Toro showed me a video on his phone: A young man with the same WARS2 mutation is doubled over in bed, frozen by intense dystonic spasms. His golden retriever is inconsolable, jumping around at his feet and barking for help. In another clip, the same man is walking upright and shooting free-throws with textbook form. The difference is dopamine, supplemented through a drug called levodopa. It doesn’t fix the underlying deficiency, but for many people with parkinsonian disorders, it can help alleviate tremors, stiffness, and muscle spasms.
Shortly after he was diagnosed, Graham began his penultimate year of public school. He also began taking daily levodopa. By the time he returned from winter break, he was walking around the house. “We hadn’t seen him walk on his own in ten years,” Susan tells me in an inpatient suite at the Clinical Center. Graham can’t cover long distances—his muscles are too rigid, tendons too short, arches too high—so he still uses his wheelchair or walker in public. Even so, he’s “racing” on the walker, putting in five laps around his school’s campus every day.
With his newfound confidence, Graham is exploring interests outside the house. He volunteers with a youth group. He recently joined a basketball team through the Special Olympics. A game against the Woodstock Fire Department is fast approaching, and Graham has been working on his fundamentals. “I jumped out of the chair and grabbed the ball the other day,” he tells anyone willing to listen.
“I just want him to be happy,” Susan says. “I want him to have things in his life that give him some fulfillment, for him to feel like he has a purpose and isn’t just putting in time.”
No doctor can say exactly how Graham’s disease will progress. As he ages, his motor function could further deteriorate. And the effectiveness of the levodopa will almost certainly wane within a few years. Eventually, they’ll need to consider deep brain stimulation, a permanent electrical implant in the basal ganglia that helps regulate movement. But for now, they’re reveling in the miracle of a slightly more ordinary life. Graham is eating meatballs. He’s getting dressed in five minutes instead of an hour. And he’s finally made peace with his oldest enemy: shoes.
Toward the end of our chat, he points to his feet and grins. “Look,” he says. His rubber clogs are gone. In their place are black high-top sneakers, the kind every high-school boy has coveted at some point. “They have laces.”
This article appears in the March 2024 issue of Washingtonian.








