In the summer of 2012, Dr. Stephen Hoffman did everything he
could to keep his mind off the experiment. He took a vacation to Malaysia
with his wife and three children. He worked out for 45 minutes a day. And
each morning, he walked into his Rockville office, sat down at his desk,
and pretended it didn’t matter what the results of the study would look
like. But as the calendar flipped to October, he found it impossible to think
about anything else.
One year earlier, researchers had begun injecting Hoffman’s
experimental malaria vaccine into healthy volunteers. Any day now, the
National Institutes of Health might call with the results.
In years past, when the critics or the failure or the anxiety
became too much, Hoffman found himself lying awake at 2 AM, his mind
spinning with panic. He’d made a lot more money before he began chasing
this vaccine a decade earlier, and nobody ever called him a crackpot. He
had a wonderful family and all the honors a scientist could hope for. He
never had to worry about a blackout at his laboratory or a grant that
might not get approved or his life’s work being measured by one last
experiment.
“I have said, ‘Why do I need this?’ ” Hoffman says. “And the
answer is that this is a gift and a treasure to have the opportunity of
making this kind of impact against the kind of odds that were presented to
me. It’s an extraordinary opportunity in life. How many people get that
chance?”
Now, as he waited to find out—once and for all—whether his
vaccine worked, Hoffman felt the pressure of a basketball player in a
playoff game. “It’s double overtime,” he says. “And you’ve got to make the
last shot.”
It’s been more than 30 years since Hoffman set out to rid the
world of malaria—the mosquito-borne, parasitic disease that has outwitted
every scientist before him. He couldn’t have chosen a more ruthless
adversary. Malarial fevers have ravaged humankind for half a million
years. There’s evidence that the disease killed Alexander the Great,
helped unravel the Roman Empire, and repulsed the army of Genghis Khan.
Malaria parasites infected George Washington and led to a million Union
casualties in the Civil War. The Centers for Disease Control and
Prevention was established specifically to fight the epidemic. Even today,
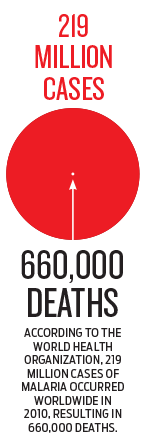
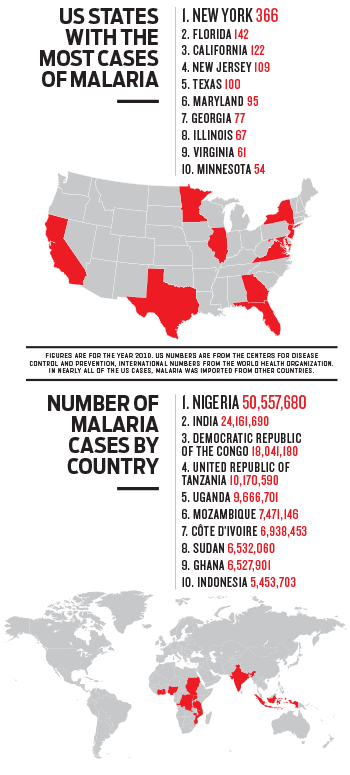
malaria afflicts nearly 220 million people a year—predominantly African
children—and kills more than 650,000.
“It’s probably the greatest single source of human tragedy in
the history of our species,” says Dr. Thomas Richie, research coordinator
of the US Military Malaria Vaccine Program.
Hoffman’s quest to annihilate the parasite has come at his own
peril. He has trudged through Indonesian swamps and watched children with
malaria die in his arms. He has injected experimental vaccines into his
body and survived a plane crash during a research trip to Kenya. He once
let more than 3,000 malaria-infected mosquitoes feast on his
arm.
But Hoffman’s biggest gamble came in 2002 when he quit his
high-paying job to develop the malaria vaccine that everyone else
considered impossible. Launching the effort from his kitchen table,
Hoffman became the laughingstock of the malaria-science
establishment.
“Even calling it a vaccine is a compliment,” Dr. Pierre
Druilhe, a malaria expert, told the New York Times. “It has no
chance of offering protection. It is like Captain Ahab in the movie trying
to kill Moby Dick with his knife.”
The NIH trial in 2012 was Hoffman’s last chance to prove that
he just might have found the way to eradicate the world’s most elusive
killer once and for all.
• • •

staff are producing what they hope will be the first FDA-licensed malaria vaccine.
The call came in around midnight. It was serious: A
four-year-old girl with malaria was slipping away, fast. Hoffman jumped
out of bed and raced through the tropical humidity to the single-story
hospital. Inside, he found the girl alive but unresponsive; her body was
cold and clammy.
It was 1984, four years after Hoffman arrived in Indonesia as a
young US Navy doctor ready to save the world. Since that time, he had seen
malaria snatch too many children from the lowland rainforests and beaches
that surrounded him. He wasn’t about to let another one go.
As with all malaria victims, the girl’s crisis began with a
tiny bug bite. When a malaria-infected mosquito pierces human skin, it
shoots a handful of microscopic parasites into the bloodstream. The
parasites squirm into the liver, where they quietly multiply. About a week
later, a violent mob of 3 million parasites explodes back into the
bloodstream, savaging red blood cells and overwhelming the immune system.
The incursion triggers fevers, headaches, chills, and fatigue. In severe
cases—like the young Indonesian girl’s—the disease chokes off blood flow
to the brain, causing coma or death.
When Hoffman and a colleague examined the girl, they found that
her blood sugar was dangerously low—a malaria-related complication that
can send patients into shock. The doctors injected her with a large dose
of sugar solution. Almost immediately, the girl’s condition improved; her
blood pressure increased, her body warmed, and she returned to
consciousness.
Walking out of the hospital, Hoffman felt like a hero. If
we hadn’t been here, he thought, that girl would have
died.
Hours later, he received another phone call. The girl was
dead.
“We had the hubris to think that somehow we were doing
something great and congratulating ourselves,” Hoffman says. “And now,
three hours later, you get a wake-up call—which is truly a wake-up
call—which is that you thought you were good, but this disease is tougher
than you are.”
Hoffman had come to Indonesia to save patients like this young
girl. During a yearlong break from Cornell University Medical College, he
had traveled to Ecuador and contracted typhoid fever. For ten days he
roasted in a South American hospital. The experience crystallized his
commitment to fight the tropical diseases—such as dengue fever and
malaria—that devastate poor communities in Latin America, Asia, and
Africa.
The Navy had one of the world’s leading tropical-medicine
programs. US soldiers have been battling malaria for as long as they’ve
deployed to the rain-soaked regions where the parasite thrives. The
parasite caused more lost person-days for Americans than bullets in
20th-century military campaigns in malaria-endemic regions. As a result,
the Department of Defense has plowed hundreds of millions of dollars into
the effort to protect US servicemembers from it.
In 1980, Hoffman had clipped his ponytail, pressed his new Navy
whites, and arrived at his post in Indonesia. But after nearly five years
of watching children die of malaria, he decided to return to the United
States to search for a way to defeat it.

Hoffman reported to the Naval Medical Research Institute in
Bethesda in 1984. By then, scientists believed they had finally outsmarted
malaria. Researchers had identified and cloned the gene in the parasite
that triggers an immune response in humans. The head of the US Agency for
International Development predicted it would take just five years to
convert this breakthrough into a vaccine. MALARIA VACCINE IS NEAR, U.S.
HEALTH OFFICIALS SAY, a 1984 New York Times headline
proclaimed.
Scientists from the Army and SmithKline Beckman—a predecessor
of pharmaceutical giant GlaxoSmithKline—scrambled to develop the vaccine.
Hoffman joined the team as a collaborator. (There are five different
species of malaria-causing parasites. Nearly all experimental vaccines
target the most deadly, Plasmodium falciparum.)
It was a thrilling time; he flew to meetings in choppers and
was whisked off the tarmac in limousines. By 1986, it was ready—malaria’s
first-ever “subunit” vaccine, so named because it used portions of the
parasites’ proteins to stimulate protection. “We all felt like we were in
the midst of history,” Hoffman says.
Scientists have never created an effective vaccine against a
parasite. The great triumphs of 20th-century vaccinology—vaccines for
polio, smallpox, measles, and mumps—all came over viruses.
Parasites are far more complex. The malaria parasite consists
of 5,300 different genes; HIV has nine. Meanwhile, malaria parasites
repeatedly change form during their multistage life cycles. “It’s like
someone who comes into a Halloween party and changes their costume every
couple of minutes,” says Captain Judith Epstein of the Naval Medical
Research Center.
Market forces have also undercut attempts to develop a vaccine.
Malaria has been all but wiped out in most Western countries since the
1950s, in part through the widespread use of DDT, and wealthy travelers
have access to prophylactic drugs that aren’t always affordable to those
living in malaria-endemic regions. Because victims are clustered in
impoverished corners of the world, pharmaceutical companies see little
financial incentive to invest in malaria research.
Hoffman was so excited about the subunit vaccine that he agreed
to test it on himself. The best way to evaluate a malaria vaccine is to
give it to a volunteer and then infect that person with malaria parasites.
If the volunteer doesn’t develop malaria, the vaccine works.

One year after he was injected with the subunit vaccine,
Hoffman walked into the Walter Reed Army Institute of Research. He was
handed a one-pint, cylindrical container with a mesh screen stretched
across its top. Inside, five malaria-infected mosquitoes
circled.
Hoffman rolled up his sleeve and pressed the container—mesh
side down—to the inside of his forearm. He felt a tickling sensation as
the mosquitoes pricked his skin. Five minutes later, he removed the
canister; an Army scientist examined the mosquitoes to confirm that each
had sucked Hoffman’s blood. Five other volunteers did the
same.
For the next several days, Hoffman and the other volunteers bit
their nails and hoped the vaccine would keep them healthy. (Those who come
down with the disease are given drugs to kill the parasites.) By day ten,
three volunteers were sick, but Hoffman and two others felt fine.
Excitement began to swell; no injected malaria vaccine had come close to
50-percent protection—and this was its very first trial. “We thought we
were going to win the Nobel Prize,” Hoffman says.
He flew to San Diego that week for a scientific meeting. During
his speech, he felt a wave of chills rush through him. By dinnertime, his
temperature had spiked to 104 degrees and his body shook uncontrollably. A
doctor examined his blood under a microscope: malaria.
The subunit vaccine had protected just one of the six
volunteers.
• • •
In 1987, Hoffman became director of the Navy’s malaria-research
program. His assignment: to develop a vaccine for military personnel that
protected at least 80 percent of its users for at least six
months.
He initially focused on making a better subunit vaccine. But
after years of frustration, he concluded that a vaccine based only on one
or two proteins would never provide sufficient protection. Unsure how else
to proceed, he revisited the landmark studies in malaria
research.
Back in the late 1960s, scientists at New York University began
zapping malaria-infected mosquitoes with gamma radiation. They found that
when these irradiated mosquitoes bit mice, they conferred malaria
protection without causing the disease. Six years later, University of
Mary-land researchers demonstrated that this same approach worked in
humans. Ninety percent of volunteers who were bitten by at least 1,000
irradiated, malaria-infected mosquitoes received protection from the
disease.
The process works by weakening—but not killing—the malaria
parasites. Once injected into the bloodstream by mosquitoes, the
irradiated parasites swim to the liver, where they induce a powerful
immune response.
There was no way Hoffman was going to ask US Marines to line up
for a thousand mosquito bites each. But he decided to repeat the
irradiated-mosquito experiments to understand the science
better.
Once again, Hoffman signed himself up for the study. In the mid
1990s, he returned to the Walter Reed Army Institute of Research to stick
another pint-size container of insects against his arm. This time, the
cylinder swarmed with hundreds of malaria-infected mosquitoes, each having
been buzzed with a dose of radiation. The bloodthirsty insects left a
circle of swollen red skin on his arm, but Hoffman didn’t stop until he
had been bitten by 3,000 mosquitoes.
Weeks later, when he was infected with malaria, he didn’t get
sick.
• • •
As his career progressed, Hoffman earned his place alongside
the field’s top researchers. He spearheaded the effort to sequence the
most dangerous malaria parasite’s genome, conducted groundbreaking studies
on experimental DNA vaccines, and published nearly 400 scientific papers.
He was elected to the Institute of Medicine of the National Academies—one
of the highest honors a scientist can receive.
Hoffman retired from the Navy in 2001 and became a top
executive at Celera Genomics, the Rockville biotech dynamo that stunned
the world in 2000 when it sequenced the entire human genome.
Although he spent his days working on cancer vaccines, there
was one project from his time in the military he still needed to finish.
Before leaving the Navy, he had assigned his staff to help him write a
scientific paper on the irradiated-parasite experiments he conducted in
the 1990s. The task took longer than expected, but by 2002 it was nearly
complete.
Thirteen of the 14 volunteers who had been bitten with
irradiated, malaria-infected mosquitoes were protected from the disease.
What’s more, the protection was long-lasting and effective against
multiple strains of malaria. No other vaccine had ever come close to those
results.
Studying the data from his office at Celera, Hoffman got to
thinking: What if he was staring at the malaria vaccine that had eluded
him for 20 years?
That March, he traveled to Keystone, Colorado, to attend a
scientific conference that attracts the world’s most esteemed malaria
researchers. One afternoon, he hosted a discussion on malaria-vaccine
development titled “Why Is It Taking So Long?”
Standing at the podium, Hoffman asked the roomful of scientists
to predict how many years it would be before an effective malaria vaccine
reached the market. His heart sank as estimates stretched nearly a quarter
century into the future.
Next, he presented the results of his irradiated-mosquito
experiments. “What should we do now?” he asked.
“Let’s use this as a model to make a better subunit vaccine,” a
researcher suggested.
“We started doing that ten years ago and we haven’t gotten very
far,” Hoffman said. “What about making this into a vaccine?”
The room fell silent.
Then, one by one, scientists stepped forward to dismiss the
idea. “People thought, ‘Oh, this can’t work,’ ” says Carole Long, an NIH
malaria researcher who helped organize the event.
No one disputed that irradiated parasites offered protection
against malaria. But getting those parasites out of the salivary glands of
a dirty, bacteria-laden mosquito and into a vaccine that would be pure
enough to meet Food and Drug Administration standards? And even if you
could get some, how could you ever produce enough for a disease that
affects more than 200 million people a year?
“The idea was so outlandish—it was like something from outer
space—that no one had ever bothered to see if it was possible,” says
Christopher Plowe, a malaria researcher at the University of
Maryland.
Hoffman was stunned by how resoundingly the leading thinkers
rejected his idea. He didn’t see any promising vaccines on the horizon.
And besides, scientists have used similar tactics—weakening but not
killing an infectious agent—to develop vaccines against diseases from
polio to yellow fever.
When Hoffman returned to Rockville, Tom Luke, a low-ranking
Navy doctor who had helped him write his irradiated-parasite paper, urged
him to give it a shot. “You do realize this is your vaccine?” Luke once
told Hoffman.
In August 2002, Hoffman resigned his high-paying position at
Celera and set out to make an impossible vaccine. Says Hoffman: “I just
came to the conclusion that somebody had to try this.”
• • •
The work began at his house in Gaithersburg. His eldest son,
Alexander, had recently moved back home after some post-college surfing in
Hawaii, and the two began knocking around ideas.
The first step was to secure funding. Each morning, he and
Alexander convened at the kitchen table to fill out grant
applications.
The new venture needed a name. The word “malaria” comes from
the Italian mal’aria, or “bad air”; the Romans believed that
malarial fevers resulted from breathing the noxious vapors that rose from
swamps. Hoffman called his company Sanaria, meaning “healthy
air.”
Good news came in July 2003 when NIH approved a $500,000 grant,
allowing Sanaria to move out of Hoffman’s kitchen into 750 square feet of
converted retail space in a Rockville strip mall, next to a carpet
wholesaler and a framing store. Dirt clouded the windows. “It was a dump,”
says Bob Thompson, an early member of Sanaria’s team.
The space appeared so unfit for scientific research that
Hoffman refused to interview prospective employees there. Instead, he met
job candidates in the offices of the biotech company where his wife, Kim
Lee Sim, was a molecular biologist.
“We’d say, ‘Well, our facility is down in Rockville and we are
still working on getting it ready,’ ” Thompson says.
The team stocked the space with used and discarded scientific
equipment. Thompson’s buddies he’d worked with at Celera gave him a deal
on outdated computers and lab supplies. “I paid them $2,000, and they let
me pull up in a truck and fill it,” Thompson says.
Understaffed and poorly financed, Sanaria began the painstaking
effort of creating the vaccine. “You’re starting with a product that you
know works—you just have to refine it and figure out how to mimic the bite
of a thousand mosquitoes in a shot or a series of shots,” Alexander says.
“It was an engineering problem, not a science problem.”
Their initial challenge was to breed sterile mosquitoes—free of
the fungi and bacteria in which the creatures naturally reproduce.
Sanaria’s researchers raised the insects in test tubes, feeding them a
diet of aseptic nutrients. At first, the mosquitoes all died. Then some
hatched with no wings. “We didn’t know anything,” Hoffman says. “It was
like every single small step we had to solve.”
With each setback, their understanding improved. Before long,
Sanaria was breeding thousands of mosquitoes a week, each perfectly
sterile. The insects consumed blood that had been infected with malaria
parasites. Hoffman bought a 14,000-pound gamma irradiator. (“We had to
close down Rockville Pike to have it put in,” he says.) Lab technicians
learned how to extract the tiny parasites out of mosquitoes’ salivary
glands. Hoffman’s wife and her team discovered how to purify the parasites
once they’d been removed. And a cryopreservation expert developed a way to
keep the parasites alive while they were stored in vials. By 2006, the
vaccine was nearly ready.
Inside the company, pressure ran high. Hoffman drove the staff
to meet tight deadlines. Employees worried that Sanaria’s facilities—which
now housed a gamma irradiator and millions of deadly, malaria-infected
mosquitoes—might break down at any moment. Blackouts occurred regularly.
Thompson once had to climb onto the roof with a blowtorch to defrost the
air conditioner. “I had terrible, terrible dreams all the time about
mosquitoes getting loose,” says Adam Richman, an early
employee.
Meanwhile, the malaria-research establishment believed Hoffman
had gone off the deep end. Tom Luke remembers sitting in the audience at a
scientific conference when Hoffman took the stage to describe Sanaria’s
work. As he spoke, top malaria researchers snickered: This will never
work.
Hoffman found himself waking in the middle of the night, his
thoughts racing. How can I scale up manufacturing without a bigger
facility? What if we under-radiated the parasites? And his most
urgent concern: How am I going to make payroll next
month?
Hoffman was on vacation in Alaska when he finalized the
negotiation with the Bill & Melinda Gates Foundation for a $29-million
grant to Sanaria. Hoffman was ecstatic. Since 2005, the foundation has
poured nearly $2 billion into malaria research, making it one of the
field’s most influential funders. The grant did more than provide needed
cash; it brought credibility to Hoffman’s endeavor.
At the foundation’s Malaria Forum in 2007, Hoffman spent nearly
an hour discussing his irradiated parasites with Bill and Melinda Gates.
Later that fall, Sanaria finished building a state-of-the-art
manufacturing facility in Rockville.
Flattering accounts of Hoffman’s work appeared in the New
York Times, Esquire, and National Geographic. But Sanaria
still had to prove the vaccine worked. In the spring of 2009, the FDA
allowed Sanaria to move further with testing the vaccine in human
trials.

In July 2010, Hoffman was in his office at Sanaria, preparing
to leave for the day, when he received a frantic phone call about his
second-eldest son. “He’s hurt, and it’s bad,” his son’s friend
said.
During his summer break from college, Hoffman’s son—who he
asked not be identified by name—had traveled to Indonesia for a
tropical-medicine program. As he walked to the beach that morning, a
motorcycle zipped past. The next thing he knew, he was on the ground with
blood spurting from his skull. It’s unclear exactly what happened; Hoffman
believes the motorcycle driver may have struck his son in the head with a
pole.
At the emergency room, Hoffman’s son showed signs of traumatic
brain injury; he was transferred to an Indonesian hospital for
neurosurgery. Hoffman and his wife immediately flew to Singapore, where
their son had been sent to recover from the operation. The family returned
to Gaithersburg a week later. But before his son could undergo
facial-reconstruction surgery, Hoffman had to drive to Bethesda for the
most important meeting of his life.
Still trembling from his son’s accident, Hoffman walked into
the conference room of a Bethesda hotel. There he greeted a delegation
from the PATH Malaria Vaccine Initiative, the nonprofit that administered
the Gates Foundation’s $29-million grant to Sanaria. PATH had called the
meeting to review the data from Sanaria’s vaccine trials and determine
whether its approach merited additional funding.
The trial results fell far short of expectations; Sanaria’s
vaccine protected just 5 of 80 volunteers. Throughout two days of
discussions, Hoffman pleaded with PATH officials to continue their support
for one more trial. But PATH executives insisted that Sanaria’s vaccine
hadn’t hit the thresholds to warrant another infusion of Gates Foundation
money. “We set up clear go/no-go standards,” PATH director Dr. David
Kaslow says. “And the ‘go’ criteria weren’t met.”
The news crushed Sanaria’s staff; even some of Hoffman’s most
loyal lieutenants wavered. Says Bob Thompson: “It was kind of like, ‘Well,
sometimes a great idea doesn’t quite make it because of circumstances
beyond its control.’ ”
For Hoffman, the days after the PATH meeting were among the
darkest since he’d begun hunting the parasite more than 30 years earlier.
“Your life’s dream just went down the tubes and your son is in between two
surgeries,” he says.
But despite the heartache, Hoffman refused to give up on his
irradiated parasites. He believed he knew what had gone wrong in the
trial.
Years earlier, NIH researchers had used monkeys to evaluate the
different ways of administering Sanaria’s vaccine. Some of the monkeys
received the vaccine as an injection through their skin, as you might get
a flu shot. Others took the injection directly into a vein. “The way you
give the vaccine had a dramatic difference,” says Dr. Robert Seder, the
NIH scientist who led the studies. “If you gave it through the skin, it
really didn’t work, but if you gave it intravenously—through the vein—it
worked much better.”
However, Hoffman’s team didn’t have the results of that study
when they designed their trial, so they injected the vaccine through
volunteers’ skin. After reviewing the NIH experiments, Hoffman became
convinced that the irradiated parasites would be protective if delivered
intravenously.
Hoffman rallied his staff with plans for a new trial. “I knew
that if I gave the right dose of these parasites in the right way, we
would get protection,” Hoffman says. “The question is: How many chances do
you get to do this?”
Pressure mounted further in October 2011 as GlaxoSmithKline
announced that its subunit vaccine candidate—known as RTS,S—protected
almost 50 percent of the African children who received it, a level well
short of the 90-percent protection that vaccines must achieve but
significantly higher than Sanaria had demonstrated.
Hoffman approached Dr. Anthony Fauci, director of NIH’s
National Institute of Allergy and Infectious Diseases, about funding the
trial.
“Rather than be the naysayer saying, ‘It’s not going to work,’
I’m saying, ‘Well, it isn’t as if you’ve got a lot of other candidates out
there that really, really look good,’ ” Fauci says.
In the fall of 2011, NIH began recruiting volunteers for the
trial.
• • •
The countdown began in October 2012, when the final set of 15
volunteers was exposed to malaria-infected mosquitoes. Twenty-three
previous volunteers had already come down with the disease, but this group
had received a higher dose of the vaccine. Officially, this final phase
lasted 28 days, but malaria symptoms typically materialize 7 to 11 days
after infection. Anytime during that period, an NIH researcher could call
to inform Hoffman that the rest of his volunteers were sick and his dream
was over. No news was good news.
Hoffman began holding his breath on day 7. By day 12, he fought
a flicker of excitement. Every day that passed without word from NIH, his
elation became harder to contain.
Three weeks into the final test, Hoffman was walking to a
meeting in downtown DC with Sanaria staffers. Right then it hit him. He
grabbed a colleague in a bear hug and together the two jumped up and
down.
“It’s day 21!”
“It’s day 21!”
When he got the official word from NIH, Hoffman called an
all-staff meeting in Sanaria’s conference room. Everyone, down to the
janitor, stood silent with anticipation. Then Hoffman told them: Six out
of six volunteers who’d received five doses of vaccine were protected, as
were six out of nine volunteers who’d received four doses. The room burst
into hoots and cheers.
The results became public on August 8, when they were published
in the journal Science. “The trial results constitute the most
important advance in malaria vaccine development since the first
demonstration of protection with [irradiated parasite] immunization by
mosquito bite in the ’70s,” Stefan Kappe, a malaria researcher at the
Seattle Biomedical Research Institute, told Nature.com.
Although Hoffman believes his vaccine will reach the market
within four years, many in the malaria community are unconvinced. He must
first reproduce his results in larger studies while demonstrating that the
vaccine can provide long-lasting protection against various malaria
strains. Sanaria has several trials slated to begin next year.
But it’s the logistical hurdles that critics consider
insurmountable. No vaccine has ever been administered through intravenous
injection, a clumsy means of delivery compared with the oral or
skin-injected vaccines typically deployed in mass campaigns. And many
argue that malaria-endemic regions aren’t equipped to store Sanaria’s
vaccine, which must be cryopreserved. “I think [the current challenges]
are more daunting than what he has already faced,” NIH’s Carole Long
says.
Ten years after Hoffman set out to make an impossible vaccine,
he must now return to the lab and figure out how he can deliver it to the
hundreds of millions of people who need it.
The skeptics don’t think he can do it.
Senior writer Luke Mullins last
wrote about the meltdown at the Tea Party group
FreedomWorks.
This article appears in the October 2013 issue of The Washingtonian.


![Luke 008[2]-1 - Washingtonian](https://www.washingtonian.com/wp-content/uploads/2017/10/Luke-0082-1-e1509126354184.jpg)