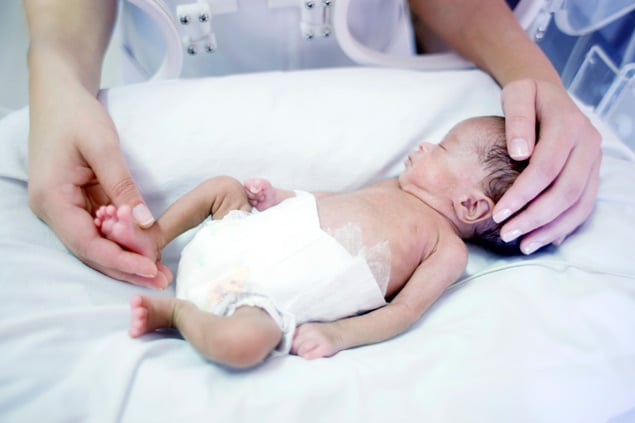
When the hospital monitor chimes again, Atticus’s parents stop talking and look up
at the screen. A light blinks an ominous red as the baby’s breathing rate falls. This
alarm lasts much longer than the previous two. Catherine and Byron spring to the foot
of Atticus’s bassinet and rub his feet. “Make sure you breathe,” Catherine tells her
11-week-old son. She and her husband keep one eye on the baby while watching the monitor.
Atticus coughs.
The chiming continues, a gentle sound at odds with the red flashes. The calm of the
neonatal intensive-care unit (NICU) can be deceptive. Just minutes ago, Catherine
explained, “You hear ‘beep beep.’ It’s really subtle, but then you see people running
down the hall and you hold your breath.”
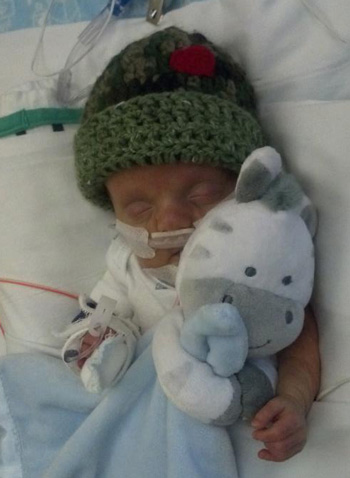
Atticus is a happy, curious baby with brown eyes and impressive blond hair currently
hidden by a cap. He likes Disney songs and stares intently at the picture books his
parents read to him. He loves Yertle the Turtle but not Horton Hatches the Egg. His
parents know this because when they read Yertle, Atticus squints happily, but when
they tried Horton, his oxygen levels dropped.
Atticus loves to be held, but he’s often not stable enough for that. If he’s having
a good day, his mother asks the NICU staff if she can pick him up. He’s so charming
that he has a following in the hospital—several nurses come to visit him before their
shifts. They’re happy to see him fight hard when they have to adjust his pressure
cuff or prick his heel, which happens a lot. They call him feisty.
He’s had to be. Atticus was born four months early, at a gestational age of 24 weeks.
His doctor at a Virginia hospital pronounced him “a highly optimistic pound and a
half” at birth; he came out crying and trying to breathe on his own.
But when Atticus was two weeks old, his intestine ruptured and he was airlifted to
this Washington-area hospital for surgery. There were so many medical personnel that
Catherine and Byron couldn’t ride with their baby. As they raced by car to the hospital,
they saw the helicopter carrying their son fly past them overhead.
“Okay, he’s coming back up—there we go,” Byron says. The chiming stops. A few minutes
later, when his mother strokes his cheek, Atticus opens his eyes for a heartbeat,
uncurls a perfect hand about the size of a quarter, smiles an unforgettable baby smile,
and settles back to sleep.
• • •
Over the last weeks, Atticus has battled six types of E. coli, his kidneys’ shutting
down, multiple infections, and heart surgery, which “he handled like a champ,” his
father says. “He’s overcome everything that’s been put in front of him.”
Except for a mind-boggling problem that Atticus’s hospital—one of the most prominent
in the country—has been powerless to solve: Atticus isn’t receiving some of the critical
nutrients he needs to survive.
Doctors and pharmacists say that because of nationwide shortages caused by a combination
of factors—manufacturing problems, a market with few incentives for companies to produce
low-profit drugs, and the government’s delayed and inadequate action—thousands of
patients are being malnourished.
There are 300 drug, vitamin, and trace-element shortages in the US, the highest number ever recorded.
Atticus’s gastrointestinal tract, like that of many NICU babies, isn’t mature enough
for digestion, so he must rely on intravenous nutrition, a formulation called parenteral
nutrition (PN), typically made up of 20 nutrients. Some babies, as well as hundreds
of thousands of children and adults, rely on PN, sometimes for months or years.
At the time of this writing—some shortages come and go by the week—Atticus’s hospital
is low on intravenous calcium, zinc, lipids (fat), protein, magnesium, multivitamins,
and sodium phosphate; it’s completely out of copper, selenium, chromium, potassium
phosphate, vitamin A, and potassium acetate. And so are many other hospitals and pharmacies
in the country, leading to complications usually seen only in the developing world,
if ever.
In Washington, for example, health professionals blame calcium deficiencies for rising
numbers of NICU babies—also called neonates—with metabolic bone disease, poor growth,
and fractures, including a baby with a broken thigh bone.
• • •
Experts call the nutrient shortage a public-health crisis and a national emergency—and
are astounded that the government and manufacturers have let the situation become
so dire.
“Children are dying,” says Steve Plogsted, a clinical pharmacist who chairs the drug-shortage
task force of the American Society for Parenteral and Enteral Nutrition (ASPEN). “They’re
not getting any calcium or any zinc. Or they’re not getting any phosphorous, and that
can lead to heart standstill. I know of a neonate who had seven days without phosphorous,
and her little heart stopped.”
“I’ve never seen anything like this in my entire career, and I’ve been a pharmacist
for 40-some years,” says Michael Cohen, president of the nonprofit Institute for Safe
Medication Practices (ISMP) and a 2005 MacArthur Foundation fellow. “This should never
be allowed to happen.”
There are 300 drug, vitamin, and trace-element shortages in the US, the highest number
ever recorded by the University of Utah Drug Information Service, which began tracking
national shortages in 2001. Approximately 80 percent of these are generic injectables,
or drugs given intravenously.
Clinicians have reported at least 15 deaths attributable to drug shortages since 2010,
and there almost certainly have been many more. There have also been serious but nonfatal complications—as
well as errors made when hospital staff substituted other drugs with which they were
less familiar. Patients have woken up in the middle of surgery, and infants have been
burned and scarred. In 2011, nine patients died and ten others developed infections
when an amino-acid shortage led Alabama hospitals to use a substitute PN that turned
out to be contaminated.
“Our patients are starving because of drug shortages. How can this happen in this country?”
There are no substitutes for vitamins, minerals, and trace elements, though. Zinc
is zinc, and without it neonates can suffer from growth and immune-system problems.
A copper shortage interferes with zinc metabolism, “which will cause white-blood-cell
production to fall to zero, and you have no cellular protection from infection,” Plogsted
says. “You need phosphorous to make energy and as an acid-based mechanism in the body.
Acidosis can prevent a child from growing, but what shows up soonest in neonates is
no energy, which equals no heartbeat. No energy is pretty much the end of the trail.”
The Children’s Hospital Association (CHA) estimates that each year at least 120,000
NICU babies need parenteral nutrition, and another 370,000 other patients receive
PN in the hospital, according to the Agency for Healthcare Research and Quality.
The nutrients in shortage aren’t rare. “We’re talking about zinc, phosphorous, calcium—trace
elements,” says CHA president Mark Wietecha. “These aren’t the latest genetically
modified drugs or something coming out of modern high-tech environments. These have
been around for decades.”
The shortages affect every patient using intravenous nutrition, but neonates are the
most vulnerable because they have no reserves. If a hospital is out of phosphorous,
for example—and many hospitals are critically low—the babies have no storage to draw
on.
“If we run out of phosphorous, there definitely will be deaths,” says a NICU dietitian
in a DC hospital. “At this point, we’re not even trying to give enough to get patients
into a normal range. We’re giving just enough to prevent them from dying.”
Some hospitals have resorted to bartering with one another to secure even a small
supply of nutrients, and many are rationing.
At least one NICU in the District is administering some trace elements only three
days a week instead of seven. At Atticus’s hospital, no patients heavier than 2½ kilograms
(5½ pounds), including NICU babies, are getting intravenous phosphorous. “You could
have a brand-new, full-term baby and they don’t qualify,” a staff member says. “There
are really sick babies and one-, two-, three-year-olds that don’t get anything at
all because we’re rationing it for our tiniest preemies.”
“It almost makes me cry—our patients are starving because of drug shortages. How can
this happen in this country?” says ASPEN past president Jay Mirtallo, a professor
of clinical pharmacy at Ohio State University. “In the last three years, there hasn’t
been one PN product that hasn’t been in short supply. I’ve traveled all over the world
talking about parenteral nutrition, and our colleagues in Europe, South America, and
Asia just look astounded and ask how this can be such a significant problem when they
have no issue whatsoever in any of their countries.”
• • •
Why haven’t you heard about the shortages? Most people haven’t. Many hospital administrators,
doctors, and even NICU nurses are unaware that patients are being shortchanged. What’s
more, several hospital staff members say that “virtually none” of the patients or
their parents know that their intravenous nutrition is so incomplete that they may
be in danger of serious deficiencies.
Atticus’s parents—who asked that their last name not be used—are an exception because
Catherine practically lives at the hospital, leaving only to sleep at a nearby Ronald
McDonald House. She has gone home just three times in three months. She hears the
nutritionists on their daily rounds, making frantic calculations. At 11 weeks, Atticus
has received no copper, chromium, and selenium since birth, and the hospital is now
out of potassium phosphate.
“These deficiencies and conditions don’t exist except in historical data or in Third World countries.”
“I feel so helpless—I want to go rob a hospital somewhere,” Catherine says. “My baby’s
been fighting and fighting, and to take away the most important things he needs so
badly, just because of a regulation? I pay my taxes! What’s the FDA doing?”
Caitlin, a NICU nutritionist in DC who requested a pseudonym, says babies are having
renal problems she’s never seen before: “I’ve noticed a lot of weird labs. I know
something’s going on, but we haven’t figured it out. These deficiencies and conditions
don’t exist except in historical data or in Third World countries.”
At Children’s National Medical Center in DC, doctors were perplexed in December 2012
when three extremely premature infants in the NICU developed a rash in the diaper
area, with blisters and bright-red lesions on their knuckles and the tops of their
feet and dark marks like lip liner around their mouths.
Doctors investigated whether the babies were reacting to diaper cream, medications,
a virus, a bacterial infection, or a new adhesive. Then, about a week after the onset
of symptoms, the physicians and a NICU dietitian put together the fact that all three
babies had been receiving PN on and off since birth—and the hospital had run out of
zinc three weeks earlier. A test confirmed that the babies were suffering from a severe
zinc deficiency, a condition that neonatologist Lamia Soghier says she’d never seen
in infants on PN before.
The hospital was able to order an emergency supply of IV zinc from Hospira, the sole
remaining manufacturer. “Immediately you could see an improvement,” says Soghier,
who submitted a field report to the Centers for Disease Control and Prevention (CDC).
“They were totally recovered about a week to two weeks after.”
A forthcoming report will detail similar zinc deficiencies in four neonates at Texas
Children’s Hospital, one of whom died of liver failure. “There are many more nationally,”
says Texas Children’s neonatologist Steve Abrams, who has been outspoken about the
shortages, unlike many other hospital personnel.
Since then, Children’s National Medical Center has been able to procure additional
zinc, according to Ursula Tachie-Menson, acting chief of the pharmacy division. For
other nutrients in shortage, such as calcium and phosphorous, Tachie-Menson says,
“we’ve called colleagues, friends, whomever, to try to get it if we’re getting very
critical—like ‘Oh, my gosh, in a week we’re going to run out.’ People have taken pity
on us. Some adult hospitals say, ‘We’re willing to spare you a few vials.’ We’re happy
when we can even get two vials.”
• • •
Health-care providers are concerned about many possible complications because of the
large variety of shortages. A selenium deficiency, for example, can lead to chronic
heart disease; a lack of chromium and copper can create neurological deficits. According
to a 2012 Journal of Clinical Oncology article, a shortage of PN multivitamins caused
several patients at St. Jude Children’s Research Hospital in Memphis to develop Wernicke
encephalopathy, a serious neurological disorder.
Hospitals are prioritizing neonates, but the shortages affect other patients, too.
At the time of this writing, Ethan, a chatty 21-month-old from Columbia, is still
at Georgetown University Hospital more than a month after having bowel surgery. Because
the hospital is short on calcium, phosphorous, and lipids, Ethan’s PN is missing them.
As a result, his phosphorous and calcium levels are so low that his mother, Emily
Greene, says his doctors are worried, and he’s losing weight that “took us forever
to gain.” He gets zinc only every other day.
“No one understands. We tell hospital officials, but even the higher-ups outside of our unit don’t get how bad it is. It’s like, who do I need to sleep with to get something done?”
“All the vitamins that normal kids get with food, our kids can’t eat,” Greene says.
“I explain to my friends and family that it’s like taking out half of a baby’s formula
or cutting out half the nutritional value in food. That’s what’s happening to our
kids. We’re unable to provide something essential for our child, and that is the most
frustrating thing for a parent. They’re already so weak, and then you weaken them
further with these vitamin deficiencies. You’re just waiting for them to get sick.”
Health professionals say they’re already seeing complications, but it could take years
to fully assess the long-term effects. “You’re creating a deficiency, and the clock
starts now,” Mirtallo says. “You don’t know when that deficiency is going to make
itself apparent, but it is going to happen.”
• • •
Caitlin, a four-foot-ten dynamo, angrily jabs a chopstick over a meal at Sushiko in
Chevy Chase. The NICU nutritionist’s dinner has more magnesium and phosphorous than
Atticus has received in his entire life.
She describes how NICU teams typically order phosphorous when a patient’s lab level
drops below 4 milligrams of phosphorous per deciliter of blood, which is considered
worrisome. But because her hospital is running low, it can’t order phosphorous unless
a patient’s labs fall to 3 milligrams or even 2. Two milligrams itself can be dangerous,
and anything below that, she says, can lead to seizures and death.
“Until there’s a big enough outcry, lawmakers and the FDA don’t care,” Caitlin says.
“No one understands. We tell hospital officials, but even the higher-ups outside of
our unit don’t get how bad it is. It’s like, who do I need to sleep with to get something
done?”
Like many other hospital dietitians and pharmacists, Caitlin spends hours on the phone
trying to discern when the next shipment of each nutrient might arrive and which ones
her unit might be able to borrow from other hospitals. She spent the entire winter
holidays figuring out dosages, calculating the minimum possible amount each of her
patients needed to survive.
One national survey found that the shortages cost hospitals hundreds of millions of
dollars a year in labor expenses, because they have to increase staffing to monitor
inventories and try to locate supplies or devise alternatives.
Often, hospital staff must make calculations quickly. “We’ve had calls saying, okay,
tonight we’re not going to have calcium. And that could be life-threatening for a
newborn,” says Sandra Robbins, a neonatal-nutrition specialist at Inova Children’s Hospital.
Even when alternatives are available, such as filtering electrolytes from an adult
dosage, using them increases the possibility of errors. Hospital staff must remember
to input the changes in every technology: the doctors’ computer system, the pharmacy
systems, automated compounders, and automated drug cabinets. Hospitals have reported
incidents in which providers forgot to update changes in an automated compounder and
gave patients a double dose of medication.
The ways hospitals stretch their supplies also may be risky. Some draw multiple syringes
from a container rather than waste any medication. “The reason we didn’t do it before
was because there was concern about contamination, entering a unit several times,”
says Keli Hawthorne, a registered dietitian at the USDA/ARS Children’s Nutrition Research
Center in Houston. “But because of the shortage, we’ve reevaluated risks and benefits
in a different way.”
Other hospitals have turned to “gray market” vendors: private, unauthorized suppliers
who acquire shortaged drugs and nutrients—or what they claim are shortaged drugs—and
sell them to hospitals at huge markups, in one case more than 8,400 percent.
“We have some real concerns about the safety of using drugs you purchase through the
gray market,” Cohen says. “How were they stored? Where did they get it from? Was it
in someone’s car? Is it counterfeit? Is it diluted down? All of these things have
actually happened.”
• • •
A crisis like this screams for a bad guy. But in this case, depending on whom you
ask, there are either no villains or several.
Many experts blame the drug manufacturers. A Department of Health and Human Services
brief suggests that because of a big increase in the number of generic injectables
coming off patent protections since 2008, manufacturers may have diverted some of
their limited capacity to higher-profit drugs.
Most of these plants have been operating 24 hours a day with only minor upgrades since
the 1960s. With just five manufacturers producing 80 percent of the injectables in
the US, when one of them suspends production to address product quality or crumbling
infrastructure, the others don’t have the extra capacity to pick up the slack.
The business model is just-in-time manufacturing: To reduce overhead, they make only
as many vials as they’re going to sell immediately. They don’t keep a backup inventory.
They don’t know what other manufacturers are producing at the same time. And when
demand outpaces supply, there’s little incentive for them to shift resources around
so they can produce more of a low-profit drug.
Generic injectables are often made eight to ten or more to a production line, in which
solutions are mixed, sterilized, filtered, and divided. That means that if a manufacturer
has a problem and must temporarily halt a line, the cessation affects all of the drugs
on that line.
Just five manufacturers produce 80 percent of the injectables in the US.
To produce more of a drug, a manufacturer must prioritize it over other products that
share the line. And if the cost to return the line to Food and Drug Administration
standards is too high, companies may simply choose to stop producing them.
In the last five years, all of the five companies that make most of the generic injectables
in the US had manufacturing problems and suspended production.
“It’s a recipe for disaster,” says Erin Fox, who directs the University of Utah Drug
Information Service. “These companies have really let the American public down by
choosing to not have a backup plan. They don’t care if they run out of something that’s
lifesaving, because to them it’s a business.”
In 2010, a chemotherapy-drug shortage was traced to three manufacturers, one of which
closed its plant. Clinics had to turn patients away because they were out of the drugs.
Public outcry led to a media firestorm, and cytarabine, the most important chemotherapy
drug to treat leukemia, returned to the market after 11 months.
But this time around, nutrients have slipped through the cracks. Policymakers don’t
understand the significance of IV nutrients, health advocates say. “It was seen as
a sexy news article when it was chemo and infants and children couldn’t get their
cancer drugs,” says Bona Benjamin, medication-use quality-improvement director at
the American Society of Health-System Pharmacists (ASHP). “But this is just as sad
or worse.”
Before Stanley Dudrick invented
intravenous nutrition in 1967, nearly all premature infants died. At the time, the dogma was that nutrition couldn’t be given entirely through an IV.
When Dudrick used PN to save a dying month-old infant, hundreds of physicians came
to see her because “to be able to grow a baby was a marvel,” he says.
PN is now considered one of the most important accomplishments in modern medicine—which
makes it all the more distressing that unnecessary shortages are reducing its efficacy.
“It breaks my heart,” says Dudrick, now a professor emeritus at Yale. “This is because
big pharma isn’t making enough money on these components. It’s tragic. I just don’t
know how they sleep at night.”
In addition to the long-term shortages, some nutrients will be in one day, out another,
because even once a manufacturer resumes production, it must replenish supplies in
hospitals across the country. Doctors and nurses come to work not knowing what nutrients
will be in stock. Kaashif Ahmad, a San Antonio neonatologist with Pediatrix Medical
Group, says that the supply is so unpredictable that when he walks into one of the
many hospitals he covers, he doesn’t know what will be missing: “It’s different at
every single institution in the same city—no lipids at one hospital, in another no
phosphorous.”
At times, senior members of the NICU staff at Inova Children’s Hospital have had to divert their attention from patient care to spend hours figuring out what
the department would do overnight without calcium gluconate. “Sick newborns often
have low calcium levels, low enough to be life-threatening if we don’t have something
to supplement them quickly,” Sandra Robbins says. “It’s very, very difficult to deal
with these shortages and having no information about how long is this going to last.”
The rationing pits patient against patient, forcing health-care practitioners to decide
who gets a critical nutrient and who has to go without. Says Mirtallo: “I can’t imagine
another time in my professional life when we were accepting the unacceptable.”
But is it the manufacturers’ fault?
A 2012 report by the House Committee on Oversight and Government Reform places much
of the blame on the FDA. The report says that because the agency ramped up inspections
and sent a flurry of warning letters, “four of America’s five largest manufacturers
of generic injectable products” simultaneously shut down 30 percent of their manufacturing
capacity.
FDA officials have responded that the increase of warning letters was only a “modest
fluctuation” that didn’t correlate with the dramatic increase in shortages.

But records show that the number of warning letters related to drugs and biologics
nearly doubled between 2009 and 2010, from 34 to 60, before falling to 48 the next
year. The recent surge in shortages began in 2010.
Manufacturers are reluctant to talk. APP Pharmaceuticals, which produces calcium gluconate,
sodium phosphate, and magnesium sulfate, and American Regent, which makes several
IV nutrients currently in shortage, both declined to comment for this article.
“There’s no upside to our guys talking about that,” says a spokesperson for a manufacturers’
association. “Even if the FDA’s doing something terrible, we can’t criticize them.
They regulate us. There’s not one cause of drug shortages. But if you call the FDA,
they’ll say it’s our fault.”
Indeed, in a July 2012 letter to Congressman Elijah Cummings, a ranking member of
the Oversight and Government Reform Committee, FDA assistant commissioner Jeanne Ireland
passed the buck. “The root causes of drug shortages . . . lie largely outside of FDA’s
purview,” she wrote. “During 2011 nearly 70 percent of all drug shortages were related
to manufacturing production problems, including quality-related issues and delays.
. . . In 2012, quality-related problems and delays have continued to account for the
majority of shortages, especially those involving sterile injectable drugs.”
Valerie Jensen, associate director of the drug-shortage staff at the FDA’s Center
for Drug Evaluation and Research, says the main cause of the current nutrient shortages
has been the shutdown of American Regent. The company had recalls of IV calcium gluconate,
dexamethasone sodium phosphate, and potassium phosphate, among other injectables,
because of visible particulates (such as glass or silicone) in the products, which
could disrupt blood flow. In 2011, the FDA sent the company a warning letter. At the
end of 2012, Jensen says, the company chose to shut down to address its problems.
Instead of reopening after 30 days as planned, as of press time it was still not fully
operational.
Hospira cites “the difficulty of ramping up production to cover for the unanticipated
loss of production from that manufacturer” as another factor contributing to the shortages,
a company spokesman says in an e-mail. Hospira makes several injectable nutrients—plus
lipids and injectable vitamin A—that are in shortage.
American Regent has begun to release small quantities of calcium gluconate and sodium phosphate, but there
are still particles inside that pharmacists must filter out. The FDA wouldn’t normally
allow these vials into the market.
“It is a risk, but the risk of not having the drugs is greater,” Jensen says. “Because
we know the filter renders the drug safe for use, we felt this was the way to get
that drug available for patients.”
• • •
The House committee report doesn’t mention the reasons the FDA cautioned the manufacturers.
At some facilities, the agency found mold on the walls, open containers of urine,
and metal chips in vials—serious problems, to be sure. An examination of FDA inspection
letters reveals dozens of additional infractions. But the report notes that a review
of the violations “did not find any instances where the shutdown was associated with
reports of drugs harming customers.”
A March 2013 committee follow-up letter—which was obtained for this article but has
not been made public—directs the FDA to turn over documents and appear for a briefing
“in order for the Committee to understand how FDA is managing the drug shortage crisis.”
The rationing pits patient against patient, forcing health-care practitioners to decide who gets a critical nutrient and who has to go without.
“It appears that FDA failed to properly balance regulatory benefits and regulatory
costs when the agency took actions that effectively shut down a significant amount
of manufacturing capacity at most of America’s major producers of generic injectable
drugs,” the congressional letter states.
The letter echoes an accusation in the original report that the FDA knew as early
as mid-2011 that its enforcement measures might lead to shortages. That’s when FDA
warning letters began to mention shortages, instructing manufacturers to contact the
agency before making a decision that would result in a drop-off in production.
Jensen says the FDA changed the language after the drug manufacturer Teva closed down
in 2010, triggering shortages of cancer drugs. “If FDA sends a warning letter, it
just tells the company what they need to correct,” Jensen says. “If they decide they
need to shut down, that’s a decision they make, but it’s something we try to avoid.”
The committee letter also accuses the FDA—based on January briefings with industry
representatives—of taking too long to provide feedback on manufacturers’ plans to
fix problems the FDA asked them to correct.
“FDA has been very slow to react and to do anything,” says Blair Childs, senior vice
president for public affairs at Premier Healthcare Alliance, a large group-purchasing
organization (GPO). “There’s a ‘we’re not the cause of the problem’ mentality.”
The manufacturers’ spokesperson says the FDA is constrained by “an enormous mandate
without enough resources. They don’t have enough trained people; they don’t have enough
money. The FDA’s always getting negative feedback for what they do wrong, and nobody’s
standing up and saying the FDA could do a better job—here’s some more money.”
Jensen disagrees: “We feel like we are adequately resourced. Our drug-shortage program
has greatly expanded. FDA has devoted great resources to this and will continue to
do so.”
• • •
Other experts blame the shortages on GPOs. Hospitals typically don’t purchase drugs
directly from manufacturers. Instead, hundreds of hospitals band together and work
with a GPO, which bids manufacturers down to the lowest price possible. Because these
consortiums have driven the prices of generic injectables so low, some say, manufacturing
them isn’t profitable.
In November, six members of Congress, led by Ed Markey, asked the Government Accountability
Office to investigate whether GPO contracting practices have contributed to the drug
shortages.
“They drove prices so far down that [when there’s a production problem] manufacturers
say, ‘We’ll look at it in another month or so,’ and then ‘another month or so,’ then
the line goes down and shortages occur because revenue isn’t enough for them to keep
up with their productions,” says Ohio State University’s Mirtallo.
But Childs disputes the idea that GPOs have the power to suppress prices to the point
where production is no longer viable—or that they would want to. “It’s a nutty accusation,”
he says. “We are going to do everything we can to drive market competition, but ultimately
we want a healthy market.”
Distributors, manufacturers, and government officials say that to solve the shortage
crisis, the stakeholders must collaborate, but it’s unclear how deep these commitments
go. “Unfortunately, the companies consider it to be a trade secret of exactly which
drugs are manufactured on what lines,” Fox says. “We’ve tried to have FDA public summits
to get stakeholders in a room. When we ask places like Hospira, Bedford, Teva, and
APP what would fix the problem and we say, ‘Do you need more money?,’ they don’t give
a straight answer.”
It’s perhaps little wonder that manufacturers are tight-lipped regarding shortages.
They view the specifics of their operations as proprietary and don’t want to frighten
off shareholders. But hospitals’ decision to hide the issue from patients, parents,
and the press is more baffling.
A Virginia Hospital Center spokesman refused to let NICU nutritionists speak for this
article. But a pharmacist at the hospital said they were short of “everything under
the sun,” completely out of selenium, and rationing products such as lipids and sodium
phosphate. “Oh, God, it’s frustrating,” the pharmacist said. “I would think this place
would have something as simple as salt and electrolytes available for patients. It
doesn’t make sense and it’s very dangerous, especially for the babies.”

Pharmacists from two other local hospitals claim that nutrient and trace-element shortages
have been resolved, but NICU employees at those hospitals say otherwise.
“We never got to a point where we ran out of anything other than zinc,” one hospital
administrator, flanked by a PR executive, told me. Another hospital employee, unchaperoned,
later called the administrator’s statement “bullshit” and said, “She flat-out lied.”
A NICU dietitian at a DC hospital says that once she alerted her hospital’s administration
to the nutrient shortage, after one meeting with the risk-management and legal teams,
she and her colleagues were shut out of further meetings: “The impression I got was
‘Don’t go to the media.’ ”
Some NICU staff members contacted for this article said they were afraid to speak
on the record because their bosses would blame them for reporting news that could
reflect poorly on the hospital.
Even when asked as simple a question as “What PN components is your hospital short
on?” a George Washington University Hospital NICU dietitian said softly, “There needs
to be attention drawn to this issue. My hands are tied.” Another employee revealed
that GW is out of calcium chloride and short on zinc chloride, magnesium sulfate,
selenium, and other items and is sequestering nutrients for the neonates.
Keli Hawthorne, the dietitian at the USDA/ARS Children’s Nutrition Research Center
in Houston, says she’s not surprised that hospitals are muzzling their dietitians
because they don’t want to scare people or attract blame. “There’s concern people
would think we’re not appropriately caring for their infant,” she says. “And the fact
is we’re doing the best that we can.”
But by remaining silent, hospitals end up doing a greater disservice to the patients
they’re worried about scaring: If people don’t know about the problem, they won’t
push lawmakers to fix it. And patients will continue to suffer.
Some people already know about the shortages, though. Tens of thousands of patients
rely on IV nutrition at home, where home-health-care companies send them supplies.
Many of these patients, or their parents, know exactly what belongs in their PN, so
they know exactly what’s missing.
Parents are desperately checking the FDA’s drug-shortage site to learn when their babies might finally get the nutrients they need—but the posted availability dates keep slipping ever farther away.
“You just kind of have to pray,” says Kristina Colmer, a mother in Midland, Maryland,
whose three-year-old daughter, Paige, has been on PN since birth. “PN is what keeps
her alive, so these shortages are terrifying us.” The family’s home-health-care company
hasn’t been able to get zinc, phosphorous, or calcium, and Paige hasn’t received IV
pediatric multivitamins in more than a year. She has been taking adult multivitamins
instead, but now those are becoming difficult to get, too. She’s getting lipids only
because she’s enrolled in a clinical study.
The girl’s phosphorous levels have been so low that she was almost hospitalized. Colmer
fears that her daughter will go into renal failure because of her lack of phosphorous.
“She’s the light of our lives, a complete joy,” she says. “I don’t know what we would
do without her. But our reality every day is we never know when the last day may be.”
Another reason hospitals may be keeping the issue out of the press is that there’s
no equitable system of drug distribution. “It’s first come, first served,” Abrams
says. “There isn’t a good national or even local system for saying, ‘X hospital has
a critical shortage of this component, Y hospital has extra—can we shift it over?’
”
Some hospitals with extra supplies hoard them to protect their patients. “We’ve started
to stockpile, which we didn’t do before,” says the Virginia Hospital Center pharmacist.
“You have to do it. You try to buy more than you would normally need, and that is
adding to the problem.”
Hospitals are afraid to share their supplies because they don’t want to reduce the
buffer for their own patients. Some have refused even Children’s National, the highest-level
NICU in the Washington region and the referral center for the sickest kids. “They’d
say, ‘We have one vial—we can’t give you our one vial,’ ” says director of pharmacy
Ursula Tachie-Menson.
Because many adverse effects aren’t getting reported, the full scope of the crisis
isn’t known, says Bona Benjamin of the American Society of Health-System Pharmacists:
“If it’s being reported routinely, I’m not seeing that. I’ve been encouraging the
pharmacists I talk to to keep track and consider doing articles in the biomedical
literature on the impact of shortages on their patient population, but truthfully,
they have their hands full just managing shortages. The fact that it’s a public-health
crisis would escalate priority.”
• • •
The FDA can’t force a manufacturer to produce drugs. But baby Atticus, Emily Greene,
and Kristina Colmer need a central oversight entity either to have that authority
or to find the means to secure them elsewhere—immediately.
In October 2011, President Obama signed an executive order charging the FDA to work
harder to address drug shortages. His speech at the time mentioned cancer drugs but
not nutrients or trace elements. Obama said, “Even though the FDA has successfully
prevented an actual crisis, this is one of those slow-rolling problems that could
end up resulting in disaster for patients and health-care facilities all across the
country.”
Nineteen months later, health advocates—and even the FDA’s Valerie Jensen—say the
crisis is here. Two congressional bills went nowhere, and although in July 2012 Obama
signed the Food and Drug Administration Safety and Innovation Act, experts say it
won’t create more drugs, just provide earlier warning of shortages.
The law says manufacturers must give FDA six months’ notice of an anticipated shortage
(or inform the FDA as soon as practicable). But an April 23, 2013, e-mail the FDA
sent in response to a patient inquiry includes a cookie-cutter paragraph that says
firms are not required to provide notice of discontinuations (except for sole-source,
medically necessary products), “nor is there a penalty for firms that do not report
discontinuations to FDA.”
Both the FDA and manufacturers say that they’re cooperating with the law, however,
and that early notifications have helped prevent shortages. They won’t say which drugs
have been saved—that’s proprietary information—but Jensen says nutrients were among
them.
There are lights on the horizon. Manufacturers are building extra capacity; Hospira
has invested hundreds of millions of dollars in improving its plants. Experts hope
smaller manufacturers can be enticed to enter the market to break up the larger manufacturers’
stranglehold. Eventually, someone may overhaul the system. Several doctors say their
hospitals would pay a markup of 50 percent or higher if they could be confident in
a stable supply of IV nutrition.
A fix isn’t that simple, however. The existing manufacturers are at capacity, and
bringing new ones online takes at least two years. “In 20 years of economics, this
is the most complicated market I have ever dealt with,” says Sherry Glied, a health-policy-and-management
professor at Columbia University. Once manufacturers finish building additional capacity,
she says, theoretically plants will stop running 24/7, there will be slack in the
system, “and you can deal with a shortage because people can run their lines an extra
couple hours and it’s fine. You won’t have this weird phenomenon of cascading shortages
where one drug goes on shortage, then that one gets fixed, then another one goes on
shortage because there’s very tight capacity.”

But any sweeping improvements will come too late to ensure that neonates like Atticus
receive the nutrients that are the foundation for a healthy life. Parents of kids
on PN, tired of Congress’s ignoring their pleas and the FDA’s bland form-letter responses,
are desperate for a call to action. Where are the innovators? Where is the urgency?
Following the anthrax attacks in October 2001, the US quickly mobilized to expand
the national stockpile of anthrax and smallpox vaccines. Now the CDC’s Strategic National
Stockpile (SNS) includes antibiotics, chemical antidotes, antitoxins, life-support
medications, IV fluids and administration kits, and enough vials of smallpox vaccine
to vaccinate every American. According to CDC documents, federal officials can deploy
SNS resources even in the instance of a single case of smallpox.
Could the SNS be expanded to include critical drugs and nutrients so the country has
a backup when those drugs go into shortage? Federal officials say the SNS is only
for public-health emergencies of national-security interest. But the stockpile already
contains a form of zinc and calcium: pentetate calcium trisodium injection and pentetate
zinc trisodium injection, which bind with plutonium, americium, and curium to speed
elimination from the body after radioactive contamination.
Another idea: Why can’t the FDA or the Department of Health and Human Services subsidize
manufacturers of generic injectables? There’s precedent. In 2009, HHS paid at least
$151 million to a pharmaceutical company for millions of doses of the H1N1 flu vaccine.
The same year, the government paid two other pharmaceutical companies $700 million
for the swine-flu vaccine.
The FDA Safety and Innovation Act includes incentives for manufacturers to develop
drugs to treat MRSA and other antibiotic-resistant pathogens as well as so-called
orphan drugs, for diseases that affect a small number of patients.
At some facilities, FDA found mold on the walls, open containers of urine, and metal chips in vials.
Health advocates say congressional action is necessary to help babies who need IV
nutrients now. In early May, Senator Al Franken sent a letter signed by 13 bipartisan
colleagues to FDA commissioner Margaret Hamburg asking her to “do everything in your
power” to fix the nutrient shortage.
Many doctors are pinning their immediate hopes on Congress’s forcing the FDA to form
a global pipeline to import an emergency supply. “I have friends in other countries
who could get me some, but that would be illegal,” one doctor says. In fact, pharmacists
note that the phosphorous Europe uses is a better product than that in the US because
it’s organic and doesn’t interact with calcium in the PN, meaning more phosphorous
could be included in the IV bag.
• • •
When Miguel Sáenz de Pipaón, a neonatologist at a prominent hospital in Madrid, arrived
in the US for a research visit, he was stunned by the nutrition shortages.
“It’s crazy,” he says. “That doesn’t happen in Europe.” He noted that the US relies
on a 25-year-old lipid emulsion, which is in shortage, while European hospitals use
a newer version that’s readily available. Rather than import the newer emulsion, the
US has left many patients without any lipids at all.
The only shortage Sáenz de Pipaón could recall in Spain occurred two years ago when
a Canadian factory stopped making trace elements. His hospital pharmacy immediately
secured the product from a Swedish manufacturer and had it for patients within two
days.
Hospital staff wonder why the FDA hasn’t already put a process in place to streamline
foreign inspections and certifications so that labs abroad can manufacture emergency
supplies on short notice.
Jensen says the FDA is working on it and that imported nutrients will be shipped soon:
“It took a long time to find companies willing to do it, mainly because they couldn’t
meet US needs and didn’t have the ability to ramp up for the US. The good news is
we’ve got different firms willing to do this for phosphates, zinc, and trace elements.
We moved as quickly as we could.”
An FDA spokesman says officials began looking into importation after American Regent’s
2012 shutdown. But that company’s recalls began in 2010, and it first suspended manufacturing
operations for more than a month in 2011.
The FDA must have been aware of problems because regulators sent American Regent a
warning letter in September 2011. Moreover, the FDA allowed the company to release
vials of potassium phosphate contaminated by particulate along with a filter in 2011.
Knowing that American Regent produced more than half a dozen nutrients critical to
neonatal health, couldn’t the FDA have searched for importation possibilities back
then, secured a backup manufacturer, and avoided the current shortages?
Instead, parents are desperately checking the FDA’s drug-shortage site to learn when
their babies might finally get the nutrients they need—but the posted availability
dates keep slipping ever farther away. For example, on January 16, 2013, the website
listed the update for potassium phosphate from Hospira as “Next delivery end January.
Estimated recovery 1Q [first quarter] 2013.” On January 27: “Next delivery end February
Estimated recovery 2Q 2013.” On March 7, “Next delivery late March. Estimated recovery
2Q 2013.” On May 9, “Next delivery May. Estimated recovery July.”
“The FDA has repeatedly told us that the shortages are short-term and that they don’t
need to import yet,” says neonatologist Steve Abrams of Texas Children’s Hospital.
“There’s been a general sense that this problem will go away if we just wait until
next Tuesday, and next Tuesday just hasn’t come for the last 2½ years.
“This is the first time in over 30 years of practicing medicine I’ve ever not been
able to give babies the IV nutrition they require. I don’t think the FDA has done
all they could in this regard. Why is this going on for so long with no end in sight?”
Alexandra Robbins is the author of six books. This article was supported by the Fund for Investigative Journalism. Editorial intern Emily Thompson contributed reporting to this article.