Marie Galloway has a hard time talking about what happened on April 22, 1995. It was a warm spring day, and Galloway, the elephant manager at the National Zoo, was doing her rounds. She noticed that Kumari, a 16-month-old Asian elephant much loved by visitors, seemed a little off. At first Galloway thought she was imagining it: One minute the calf seemed lethargic and uninterested in food; the next she was the sweet, sometimes mischievous toddler everyone had grown to love.
Galloway went home with nagging concerns but tried to put them out of her mind. She thought it might be a bout of colic.
The following day, a Sunday, Galloway went to the zoo to check on Kumari, expecting the elephant to be back to normal. “I mostly did it to calm my own nerves,” she says.
But Kumari had gotten worse—now she was barely eating and seemed more sluggish than the day before. Veterinarians drew blood but couldn’t find an answer. Galloway and the other keepers focused on keeping Kumari moving while Shanthi, Kumari’s 20-year-old mother, stayed close. Galloway recorded in the keeper’s log that Shanthi was “frantic” to be with her baby.
Over the next three days, the veterinary staff monitored Kumari. Everyone looked on, baffled, as the elephant’s condition worsened. Kumari wouldn’t nurse, her legs and trunk started to swell, and she had a fever. Whenever she tried to lie down, Shanthi made her stand up, using her own body to stabilize the 1,100-pound calf.
On Tuesday, Galloway and her colleagues decided to spend the night. They kept watch around the clock and tried to get Kumari to take vitamins.
Around lunchtime the next day, Galloway noticed that Kumari’s tongue had turned purple. She called the vets; the doctors, she was told, would be there right away. In the meantime, Galloway took Shanthi and Kumari outside to spend time with Ambika, one of the zoo’s older elephants. As they had every day for months, visitors gathered at the fence, excited to see the zoo’s young celebrity.
“The next thing I know, Kumari lies down,” says Galloway. “It was something she never would have done in front of Ambika, and Shanthi never would have allowed it.” Kumari was still new to the herd, and because the other elephants hadn’t yet accepted her, Shanthi was protective. “I remember looking over at Shanthi and seeing her turn and walk away,” Galloway says. “I knew it was over.”
Kumari managed to get up one last time and walk a few feet before collapsing. She died in the yard with keepers and visitors looking on, stunned.
Next: Inside the necropsy building.
The National Zoo’s veterinary pathologists are the detectives charged with solving medical mysteries. The path lab, as it’s called, is housed in a bunker-style building carved into a hill in Rock Creek Park. Inside, hallways of tan cinder block are covered in posters and charts describing the scientists’ work, complicated findings boiled down to colorful lines and drawings.

Scientists here sit bent over microscopes at black countertops—it feels more high-school biology than government-funded science lab. They spend hours a day looking at blood, tissue, and fecal samples from creatures that have rarely if ever been studied in such detail. Their job is to diagnose health problems in live animals and to determine what killed an animal.
Dr. Tim Walsh runs the department. He’s the third person to hold the position since the National Zoo hired its first pathologist in the 1960s. Today this is one of only seven zoo-based pathology programs in the country.
“You’re dealing with creatures that might have never been looked at before,” Walsh says. “It’s a lot of investigation, a lot of discovery, and you get a lot of answers.”
Walsh earned his doctorate at the University of Missouri and in 1993 began a zoo-pathology residency in Chicago. When he graduated, there were no zoo jobs available, so he took a pathologist position at Washington State University’s veterinary college, working on domestic animals, birds, and fish. He did a stint in the Galápagos studying avian diseases, then came to the National Zoo as head pathologist in 2006.
Walsh calls himself an animal coroner—his only hands-on work with zoo animals comes after they’ve died. But like a heart surgeon, he’s always on call, sometimes rushing to work in the middle of the night to begin a necropsy, an animal autopsy. He says time is of the essence when tracking a microscopic killer: “The best information comes from a fresh specimen.”
On average, four to five animals die at the National Zoo every week. While lions, tigers, and other large mammals die infrequently, the zoo’s collection of roughly 2,000 creatures includes species with much shorter life spans, such as fish and insects. (While the zoo weathered controversy over seemingly preventable animal deaths under the leadership of former director Lucy Spelman, today’s death rate is about normal for the size and breadth of its collection.) Walsh and his team perform necropsies on every one.

From the outside, the necropsy building looks like a storage facility. An accordion-style garage door creates an opening to get large animals, such as elephants, inside. A truck can back up to it, and a built-in crane can lift and move animals onto the operating table.
Like any operating room, this one is cold and sparse. There’s nowhere to sit and nothing built for comfort; the concrete floor and stainless-steel table are meant to withstand post-op hose-downs. The doctors’ tools are kept at arm’s length: delicate forceps and tiny scalpels for small creatures, a band saw for large ones.
A necropsy is a meticulous process. Zoo pathologists approach each with the same goals: to understand what killed an animal, to identify whether the cause of death poses a threat to other animals, and to collect samples for further research. While human and domestic-animal pathologists are trained to look for misshapen blood cells, enlarged organs, or anything else out of the ordinary, zoo pathologists have to approach exotic animals with open minds, figuring out what’s normal before looking for oddities.
“We venture into uncharted territory,” Walsh says. “We make comparisons with more-studied animals when we can, but sometimes we’re literally writing the book.”
When a necropsy is complete, the zoo keeps samples and specimens for its records (“We’re Smithsonian—we don’t throw anything away,” says Walsh) and offers remains to other Smithsonian branches. The National Museum of Natural History usually takes most of them, using skulls and skins in their exhibits and blood samples for DNA research. To give out the rest, the department consults what it calls the Carcass Wishlist, a database of tissue requests from researchers at non-Smithsonian institutions. There were 884 requests at last count; most are for elephants.
Next: The future doesn't look good for Asian elephants.

By all measures, the future doesn’t look good for Asian elephants. The International Union for Conservation of Nature and Natural Resources’ Red List, which tracks the global inventory of plant and animal species, has categorized the species as endangered since 1986. Its latest report says that wild populations have dropped by 50 percent in three generations. And while the IUCN puts the total number of wild Asian elephants between 40,000 and 50,000, some experts think fewer than 30,000 could remain. By comparison, there are about 500,000 African elephants in the wild.
The IUCN’s Asian-elephant Web page reads like a wistful history report, tracking where the elephants used to roam and how they once behaved in their natural surroundings. It cites habitat loss, poaching, and human mismanagement as the greatest threats. Before long, conservationists fear, a paper trail might be all that’s left.
For Marie Galloway, it’s sobering to think that future generations might never know the gentle giants she cares for. More than most, she understands just how smart and docile they can be. Research shows that elephants have complex brains capable of solving problems and learning new skills. They’ve been known to show empathy and altruism and to mourn a herdmate’s death, pausing over a carcass in a moment of silence.
As the number of Asian elephants in the wild declines, the future of the species depends on the captive population. Because of the stakes, the captive-elephant gene pool is monitored to keep it free of inbred mutations. Mating is orchestrated—and in some cases, engineered—to produce genetically optimal results. And diseases such as Kumari’s killer are pursued by experts around the world.
The National Zoo has been a leader in animal conservation for decades. In the 1960s, as most zoos were just beginning to shed their amusement-park identities and rebrand themselves as science-based institutions, the National Zoo had already hired its first full-time veterinarian. In 1965, it established the Zoological Research Division, a unit charged with studying the reproduction, behavior, and ecology of zoo animals.
Today the zoo’s elephant researchers are attacking the population problem on numerous fronts. They’re using satellites to study the movement patterns of wild elephants and developing noninvasive techniques to track individual animals. They’re also studying elephant reproduction and exploring artificial mating methods to improve birth rates in zoos.
Zoo pathologists are doing their part, too. “We help animals not as individuals or as pets but as a species,” Walsh says. “By identifying what killed one animal, we can apply that knowledge to an entire population and help make sure the whole species survives.”
Marie Galloway stood with Shanthi and watched the scene unfold: Keepers came running. Vets rushed to the yard. CPR. A crash cart. For 30 minutes they tried to resuscitate Kumari.
Since birth, Kumari had drawn crowds. She was the first elephant calf born at the National Zoo, a shining example of conservation and hope for a species facing extinction.
“We built grandstands inside the building and created a queue to control the people coming through to see her,” says John Lehnhardt, the elephant curator at the time. “Kumari was the biggest thing happening.”
Even her birth had been an event. Galloway and a colleague had stayed overnight at the zoo to monitor the labor. Thinking they had a while to go, they left to get dinner. But by the time they came back, the calf was already in the birth canal.
Galloway called Lehnhardt and told him he’d better come quickly. “I lived in Northern Virginia and made it to the zoo in 25 minutes,” he says. “Within five minutes, we had Kumari.”
The staff was overjoyed: The baby seemed healthy, and Mom was calm. But when Kumari tried to stand up, she fell and let out a yelp. The noise startled Shanthi, who had never been around a baby elephant. She went with her strongest instinct—to protect her herd against an intruder—and stepped hard on Kumari’s head.
What had been a moment of joy turned to panic. Although Kumari seemed to recover, it was the first dip in a long roller coaster of health problems: Kumari’s lack of weight gain, a weak immune system, a blood transfusion.
“She never thrived,” Galloway says. “She was a sweet elephant but never the robust thing she should have been.”
Galloway and her colleagues had just started to feel they were out of the woods when Kumari died. Her death felt like a movie that ended too soon. But for the National Zoo’s pathology team, it was the beginning.
Next: What they found inside Kumari's body.
Dr. Richard Montali, then the zoo’s head pathologist, got a call from a vet relaying the bad news: Kumari had died, and a truck was on its way with her body. Montali assembled his team to get the necropsy under way as soon as possible.
Dr. Laura Richman was 15 minutes away at the Armed Forces Institute of Pathology at Walter Reed Army Medical Center. She was completing a two-year pathology residency at the National Zoo but was doing some training and course work at Walter Reed. Montali told her to come quickly.

About a dozen doctors worked on Kumari through the night, examining every inch of her inside and out. They came to the table with a list of suspects—poisoning from toxic plants, septic shock from a bacterial infection—but none fit. Like crime-scene investigators, they documented everything, taking notes, pictures, samples.
What they saw was shocking: thousands of tiny hemorrhages in Kumari’s heart, liver, tongue, and intestines. The evidence suggested the presence of a viral or bacterial organism, but they couldn’t be sure until they got the samples under a microscope.
Montali sent the samples to be made into slides. It took two days because the tissues had to undergo a chemical treatment to preserve them. “It was the longest 48 hours of our lives,” Montali says.
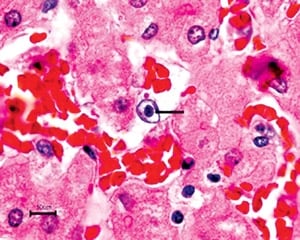
When the slides came back, Montali gathered his team around a five-headed light microscope. Richman spotted something in Kumari’s cells: tiny globs called inclusion bodies, a sign of a viral infection. The inclusion bodies were found in cells lining Kumari’s blood vessels. That explained her purple tongue and internal bleeding: The pathogen appeared to have ruptured blood-vessel cells, which caused capillaries to leak and resulted in hemorrhaging.
Richman pored through science journals to see if anyone else had reported mysterious elephant deaths. One case piqued her interest: A circus elephant in Switzerland had died unexpectedly, and scientists found inclusion bodies just like Kumari’s. They had looked at samples under an electron microscope, which offered millions of times greater magnification than the zoo’s microscopes, but they were never able to come up with a diagnosis.
Working alone one night at Walter Reed, Richman fired up an electron microscope and put in one of Kumari’s samples. She noticed dark, round objects with a dense core, which resembled a herpesvirus—a clue. “I was so excited I nearly fell out of my chair,” she says. “I think I called everyone on earth.”
If the virus proved to be herpes, it was unlike any other herpesvirus known to science. In other animals, including humans, herpes resides in nerve and epithelial tissue, such as skin, rather than in blood vessels. And it usually results in blisters and sores, not death.
With the help of John Lehnhardt, who had connections with elephant curators at zoos around the world, Richman turned up more than a dozen undiagnosed elephant deaths and was able to get tissue samples to look at. Nine of those elephants had died from the same virus that killed Kumari.
To confirm the diagnosis, scientists needed to compare the DNA of the virus to that of known herpesviruses. Because efforts to grow the elephant virus proved unsuccessful, Montali and Richman turned to plan B: a polymerase chain reaction (PCR)—a molecular technique in which trace amounts of DNA are copied over and over to yield quantities large enough for analysis.
For a year, Montali and Richman searched for someone with the right tools to run the PCR. “We went around with a little dog-and-pony show to see if we could find other herpes virologists who could help,” says Montali. The problem was, most were busy studying known herpesviruses and had no need—or time—to look for new ones.
Then Montali met Seattle-based virologist Richard Garber, who offered to help. Within a week, Garber called Richman to say that the PCR worked—the viral DNA had been successfully copied.
Garber ran the DNA through a database to search for matches. Sure enough, the herpesvirus was a hit. A comparison showed enough similarities to prove that Kumari’s killer was part of the herpes family but that this virus was a new branch of the tree. Closer analysis revealed there to be two distinct species of the virus in elephants, a version that’s fatal in Asians and another that kills Africans.
Armed with confirmation of her discovery, Richman completed her PhD work on the virus, which became known as the elephant endotheliotropic herpesvirus, or EEHV. In 1999, Richman, Montali, and eight colleagues published a paper in Science describing the virus and its effects.
“We thought we had it all figured out,” Richman says. “Little did we know we’d hardly scraped the iceberg.”
On a recent morning, Erin Latimer stands at a counter in the zoo’s National Elephant Herpesvirus Laboratory—a department the zoo established as part of the pathology lab, anticipating that EEHV would be a major threat to the species. Latimer uses a dropper to transfer elephant blood from one vial to another.
She receives blood samples from zoos and circuses across the country and runs a four-hour test to determine whether or not an elephant is sick. When a test comes back positive, she has a difficult phone call to make.
“I’ve learned a bit of bedside manner, but it never gets easier,” she says. “Telling someone their elephant has herpes feels like a death sentence.”
It turns out EEHV is responsible for about half of the deaths of young Asian elephants in the United States. So far, 37 cases have been identified, including 28 deaths and 9 survivors. In 2006, researchers reported the first known case of EEHV in a wild Asian elephant in Cambodia; more than a dozen cases have been reported since then. The threat to African elephants appears to be far less, which is why most of the research has focused on Asians. Researchers have found only three cases in captive African elephants and none in wild ones.
Without a blood test, EEHV is almost impossible to diagnose. As with Kumari, the early symptoms are vague—lethargy, loss of appetite, swelling of the joints—and most sick elephants are simply described as seeming “off.” To be safe, vets get tests at the first sign of abnormal behavior.
If an elephant is sick, the disease moves rapidly, circulating through the bloodstream and damaging vital organs. Treatment with the human antiviral drug famciclovir has seen some success, but there are no guarantees: Scientists are unsure why the drug works in some cases but not in others.
“There’s not a lot of bigtime science going on with this, so we still have a lot of unanswered questions,” says Dr. Gary Hayward, a human-herpesvirus expert at Johns Hopkins who also studies the elephant virus. He says fewer than a dozen groups worldwide are working on the problem.
Hayward has been involved in EEHV research since Richard Montali and Laura Richman got him interested in the mystery soon after Kumari’s death. Hayward invited Richman to complete her PhD work in his Hopkins lab, and he is one of the authors of the Science paper.
In the 12 years since the paper was published, experts have uncovered 14 distinct herpesviruses in elephants. Five are known to cause the hemorrhagic disease, but one type—EEHV1—is responsible for 90 percent of the deaths.
“Herpesviruses are supposed to be well behaved, quiescent viruses that are well adapted to the host,” says Hayward. “The big question here is why some of these are not.”
Elephants can carry more than one species of the virus, including the deadly ones, and survive. Most deaths have been in calves between ages one and eight. Experts theorize that antibodies in the mother’s milk protect them during infancy, but once a calf is weaned, it’s up to the animal’s immune system to provide protection.
Why do some young elephants die while others live? Hayward thinks the answer might lie in the timing: If an elephant first contracts one of the nonlethal types, the immune system might be stronger and better equipped to combat the more dangerous viruses later. But if a young elephant is initially exposed to a lethal virus, it’s likely its body won’t be able to fight off the disease.
Next: How to fix it—that's the question.

Experts recently discovered that elephants sometimes secrete and shed the virus in their trunks, but there’s no proof it’s transmitted that way. “We think it’s likely transmitted in bodily fluids, like saliva—most herpesviruses are,” says Hayward. “That said, it’s very unlikely that it’s passed through placenta, and there’s no evidence for it in semen.” That’s good news for those who fear that elephant-breeding programs put calves at risk. And with evidence that EEHV is a wild-born rather than captive-born disease, calves in zoos are at no greater risk than wild ones.
Scientists think it’s likely that all African elephants carry—but don’t die from—at least three viruses that are similar to EEHV1, the type that frequently kills Asians. When Richman first published her paper, the zoo community feared that the cohabitation of Africans and Asians in zoos meant that Africans were passing the virus to Asians. But when Asian elephants that had never been exposed to Africans—including wild calves in Asia—began dying from EEHV1, experts had to search elsewhere for a cause.
Hayward looks to evolution for an answer. Scientists have compared the DNA of all the EEHV types to the DNA of other herpesviruses. They’ve found that the EEHVs formed a distinct branch in the herpes family tree about 100 million years ago. They branched into the different EEHV types about 35 million, 20 million, and 10 million years ago.
Says Hayward: “Asian and African elephants became separate species between 4 million and 7 million years ago,” he says, “so it looks like the viruses have been with elephants from the beginning.”
Hayward believes the viruses were relatively harmless until at least 10,000 years ago. Then, he suggests, African elephants may have come into contact with Asians and passed along the deadly form of the virus. In that time, Asians and the virus simply haven’t evolved enough to adapt to each other.
“It’s not good for a virus to kill its host; that’s not how it’s supposed to work,” Hayward says. “Somewhere along the line, there was an accident. Now we’re having to deal with how to fix it.”
How to fix it—that’s the question.
The obvious solution is to create a vaccine, but attempts to grow the virus in cultures—the first step in developing a vaccine—have been unsuccessful. There are ways to make vaccines without growing the virus, but those are usually less effective. Still, Richman says, they would be better than nothing.
Because only a few hundred elephants would get the vaccine, it’s not a profitable endeavor. Richman spent years pitching the idea to drug companies, but none would do it. More recently, a few academic institutions have expressed interest.
Other research efforts are getting at the problem from different sides. Last year, scientists at Baylor College of Medicine developed a test that can detect EEHV1 more quickly and in smaller quantities than the National Zoo’s method. It’s also better able to identify false positives and can pick up traces of the virus in healthy elephants that are secreting it in their trunks—something no other test has been able to do.
“It seems that the earlier an elephant begins treatment, the higher its chance of survival,” says Dr. Paul Ling, one of the lead researchers at Baylor. “Our test shortens the time it takes to get a diagnosis and can detect the virus even before symptoms are apparent.”
Baylor researchers are also developing methods to understand the genomic structure of EEHV—methods that could, Ling says, have future payoff for decoding new human viruses and how they work.
Tim Walsh hopes to bring Baylor’s test to Washington this year. He’s submitted a grant proposal to cover the cost: $20,000 to $30,000 for the machine plus supplies and upkeep.
In the meantime, the National Zoo is focusing on other EEHV research. Erin Latimer is testing the blood serum from healthy captive elephants and looking for antibodies to EEHV1. Her project will map which elephants have been infected with what virus and may provide clues to how elephants fight off disease. There’s talk of banking the antibody-rich serum and using it as treatment.
Latimer also plans to work with researchers overseas to better understand treatment options. “We don’t know why famciclovir seems to work in some cases but not in others,” she says. “Is it the drug that’s working, or is it the supportive care—fluids, supplements—that’s turned these elephants around?”
Another zoo initiative: coordinating with researchers to get a better handle on herpesviruses in wild elephants. A 2009 report by the International Elephant Foundation said that the virus had been found in wild elephants in India, Cambodia, and Thailand. At least eight Asian-elephant calves had died from the disease. Such findings lend credence to Hayward’s evolutionary theory, but they don’t prove it. “The answer will come from looking at which EEHV types are in wild African elephants and which are the naturally evolved, adapted types in Asians,” he says.
Two years ago, Latimer helped Dr. Arun Zachariah, a veterinarian in Kerala, India, set up the first EEHV field lab. He became interested in the research after performing necropsies on wild elephants that had died suddenly. Like the lab at the National Zoo, his is kept busy by veterinarians who send him samples to test. Last year, Latimer brought supplies to a zoo in South Africa in hopes of getting a lab going there, too.
“We need to learn about the virus and how it works in its natural hosts so that we can better take care of our zoo animals and protect the species as a whole,” says Latimer. “But with so many unanswered questions, making that happen is proving to be another story altogether.”
It’s a cold winter morning, and snow has started to fall at the National Zoo. You wouldn’t know it standing inside the new elephant barn: It’s 76 degrees in here.

The barn opened last September as part of the new Elephant Trails habitat, a testament to the zoo’s commitment to the species. In addition to the barn, the first phase of the expansion includes two outdoor yards, a quarter-mile elephant walking trail, and a courtyard with interactive exhibits for visitors. Phase two, slated for completion in 2013, will include an “elephant community center” where the animals can socialize as they would in the wild. The total cost—$52 million—makes it one of the largest undertakings in the zoo’s history.
The barn was built to house eight or more elephants, but Shanthi, Ambika, and Shanthi’s nine-year-old son, Kandula, are currently the only residents. The place feels palatial: It has two-story ceilings with an observation deck; five “suites,” or stalls where the elephants sleep, eat, and bathe; and a crane-like Elephant Restraining Device, which vets use for health exams.
The stalls are cordoned off by steel bars that can withstand up to 15,000 pounds of force. Kandula likes to reach his trunk between them, touching the keepers as they walk by.
Galloway and the other keepers no longer go into the stalls with him—they say he's too powerful and rambunctious. Though he just wants to play, he might bat at them with his trunk and deal a blow that could knock the wind out, or worse.
“In a way, it’s good to have that distance,” Galloway says. “After Kumari died, I built a shell around my heart. It was hard to come back from that.”
Kandula’s birth in 2001 was a big step in the healing process. Since then, Galloway has been the animal’s primary trainer. Not yet fully grown, Kandula weighs about 5,900 pounds and is nearly eight feet tall, but Galloway can get him to kneel, lift a foot, or walk in a circle at her command.
Part of the training was designed to acclimate Kandula to getting regular blood tests for herpes. There have been a few scares—days when he’s seemed “off,” just like Kumari, sending zoo vets and pathologists into a flurry of action—but so far he’s virus-free.
The zoo staff remains cautiously hopeful that the species and the herd at the National Zoo will grow and prosper in the face of a mysterious killer. That $52-million price tag for the new elephant habitat is more than an investment; it’s an act of defiance.
“If you want to make a commitment to a species, you have to take on all its challenges, even the scary ones,” says Tony Barthel, the elephant curator. “Herpes is one of those challenges. We can’t stop because we’re afraid.”
This article first appeared in the March 2011 issue of the Washingtonian.


















